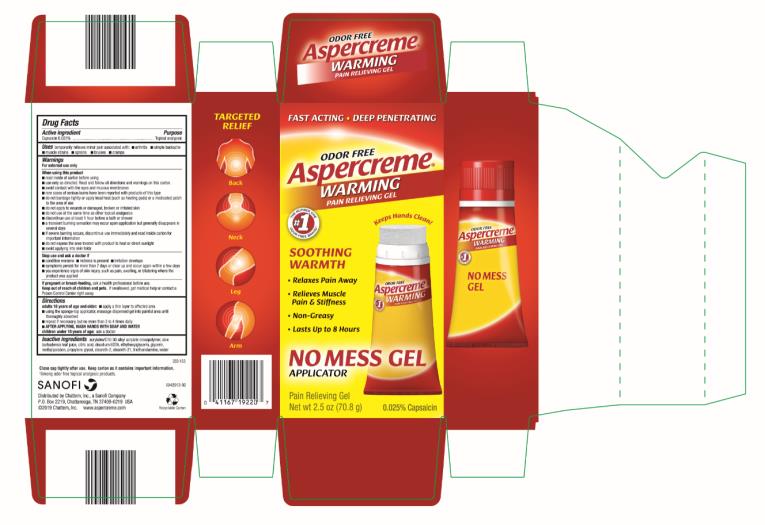
ASPERCREME WARMING NO MESS- capsaicin gel
Chattem, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Aspercreme No Mess Warming Gel
Uses
temporarily relieves minor pain associated with: ■ arthritis ■ simple backache ■ muscle strains ■ sprains ■ bruises ■ cramps
Warnings
For external use only
When using this product
■ read inside of carton before using
■ use only as directed. Read and follow all directions and warnings on this carton.
■ avoid contact with the eyes and mucous membranes
■ rare cases of serious burns have been reported with products of this type
■ do not bandage tightly or apply local heat (such as heating pads) or a medicated patch to the area of use
■ do not apply to wounds or damaged, broken or irritated skin
■ do not use at the same time as other topical analgesics
■ discontinue use at least 1 hour before a bath or shower
■ a transient burning sensation may occur upon application but generally disappears in several days
■ if severe burning occurs, discontinue use immediately and read inside carton for important information
■ do not expose the area treated with product to heat or direct sunlight
■ avoid applying into skin folds
Directions
adults 18 years of age and older:
■ apply a thin layer to affected area
■ using the sponge-top applicator, massage dispensed gel into painful area until thoroughly absorbed
■ repeat if necessary, but no more than 3 to 4 times daily
■ AFTER APPLYING, WASH HANDS WITH SOAP AND WATER
children under 18 years of age: ask a doctor
| ASPERCREME WARMING NO MESS
capsaicin gel |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Chattem, Inc. (003336013) |