GONAK HYPROMELLOSE- hypromellose 2906 (4000 mpa.s) and hypromellose 2906 (50 mpa.s) solution
Akorn
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
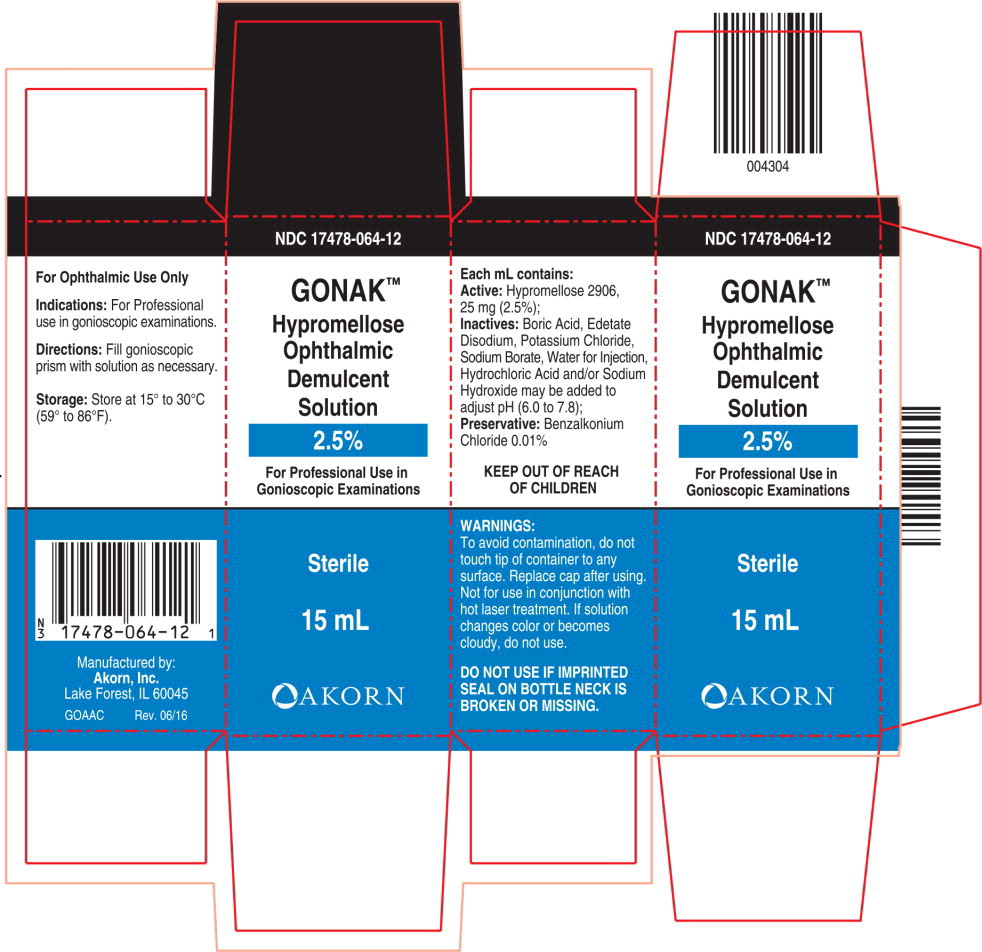
GONAK™
Hypromellose Ophthalmic Demulcent Solution
2.5%
WARNINGS:
To Avoid contamination, do not touch tip of container to any surface.
Replace cap after using.
Not for use in conjunction with hot laser treatment. If solution changes color or becomes cloudy, do not use.
DO NOT USE IF IMPRINTED SEAL ON BOTTLE NECK IS BROKEN OR MISSING
Inactives: Boric Acid, Edetate Disodium, Potassium Chloride, Sodium Borate, Purified Water, Hydrochloric Acid and/or Sodium Hydroxide may be added to adjust pH (6.0 to 7.8).
Preservative: Benzalkonium Chloride 0.01%.
| GONAK HYPROMELLOSE
hypromellose 2906 (4000 mpa.s) and hypromellose 2906 (50 mpa.s) solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Akorn (117693100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696840 | MANUFACTURE(17478-064) , ANALYSIS(17478-064) , PACK(17478-064) , LABEL(17478-064) , STERILIZE(17478-064) | |