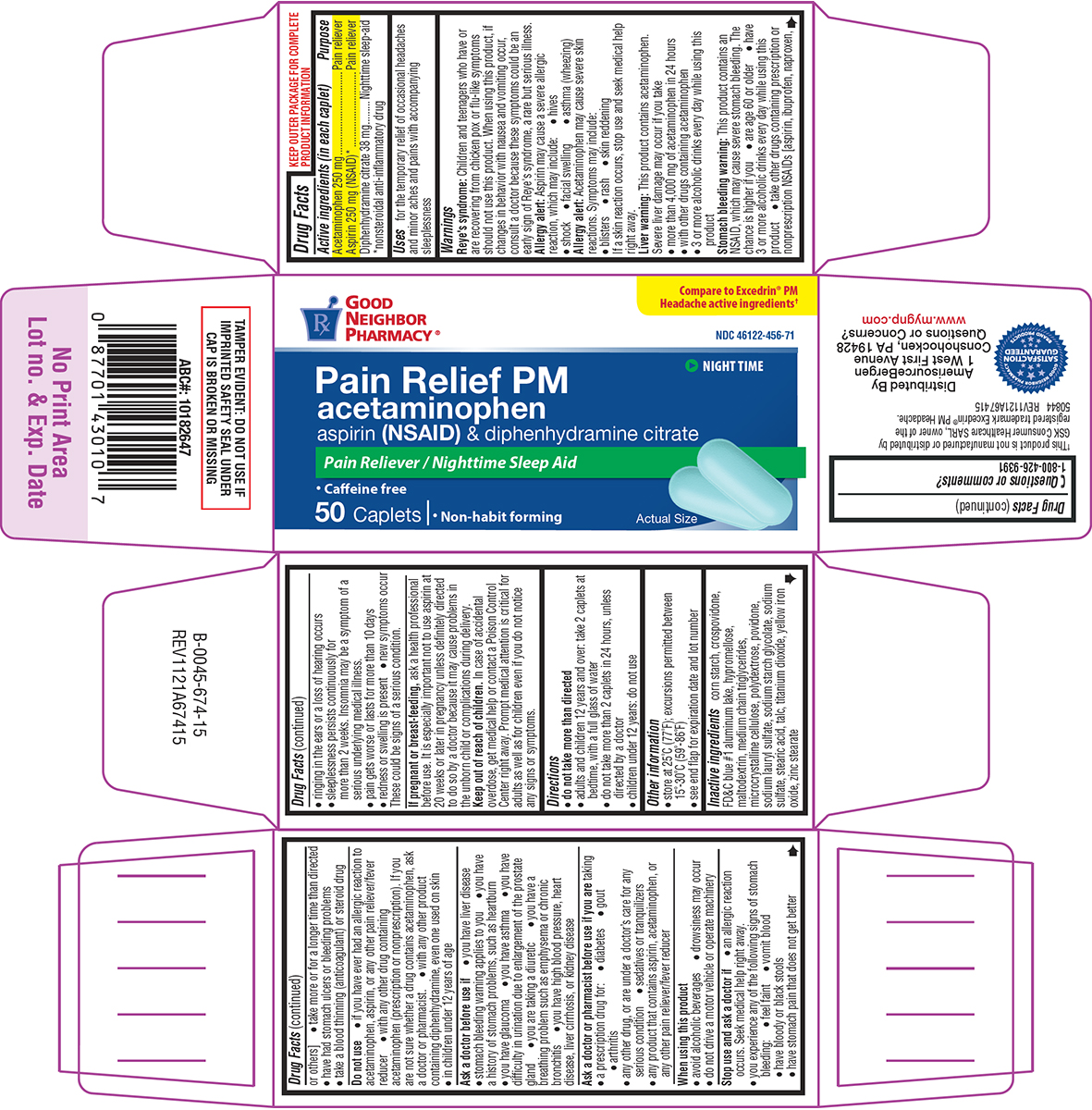
Label: PAIN RELIEF PM- acetaminophen, aspirin, diphenhydramine citrate tablet, film coated
- NDC Code(s): 46122-456-71
- Packager: Amerisource Bergen
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 4, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each caplet)
- Purpose
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- hives
- shock
- facial swelling
- asthma (wheezing)
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have 3 or more alcoholic drinks every day while using this product
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- take more or for a longer time than directed
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
Do not use
- if you have ever had an allergic reaction to acetaminophen, aspirin, or any other pain reliever/fever reducer
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on skin
- in children under 12 years of age
Ask a doctor before use if
- you have liver disease
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have glaucoma
- you have asthma
- you have difficulty in urination due to enlargement of the prostate gland
- you are taking a diuretic
- you have a breathing problem such as emphysema or chronic bronchitis
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
Ask a doctor or pharmacist before use if you are taking
- a prescription drug for:
- diabetes
- gout
- arthritis
- any other drug, or are under a doctor's care for any serious condition
- sedatives or tranquilizers
- any product that contains aspirin, acetaminophen, or any other pain reliever/fever reducer
When using this product
- drowsiness may occur
- do not drive a motor vehicle or operate machinery
- avoid alcoholic beverages
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts for more than 10 days
- painful area is red or swollen
- new symptoms occur
- ringing in the ears or a loss of hearing occurs
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of a serious underlying medical illness.
These could be signs of a serious condition.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
GOOD
NEIGHBOR
PHARMACY®Compare to Excedrin® PM
Headache active ingredients†NDC 46122-456-71
Pain Relief PM
acetaminophen
aspirin (NSAID) & diphenhydramine citratePain Reliever / Nighttime Sleep Aid
•Caffeine free
50 Caplets ι • Non-habit forming
Actual Size
TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER
CAP IS BROKEN OR MISSING†This product is not manufactured or distributed by
GSK Consumer Healthcare SARL, owner of the
registered trademark Excedrin® PM Headache.
50844 REV1121A67415
Distributed By
AmerisourceBergen
1 West First Avenue
Conshohocken, PA 19428
Questions or Concerns?
www.mygnp.comGOOD NEIGHBOR PHARMACY BRAND PRODUCTS
SATISFACTION GUARANTEED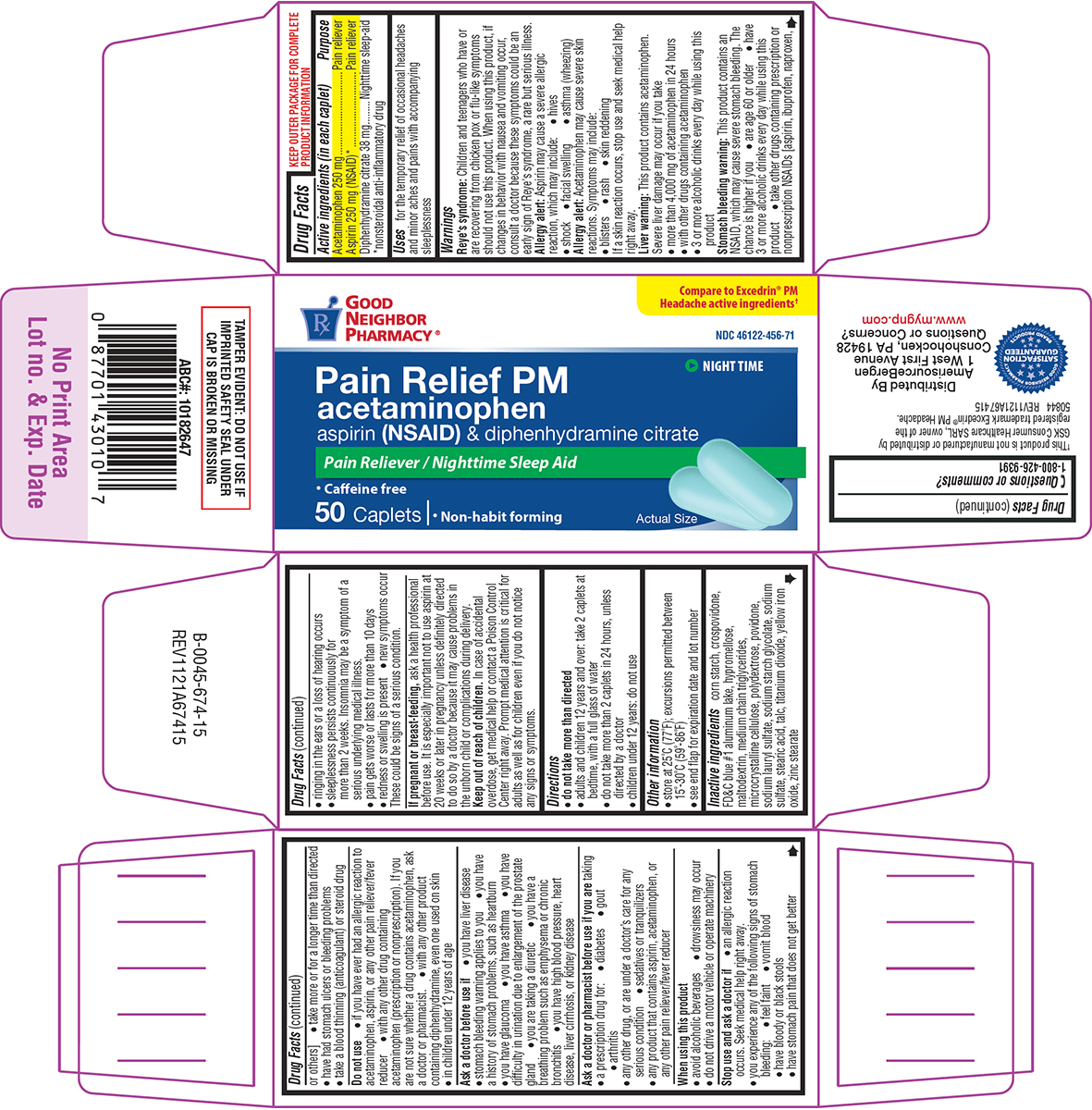
Good Neighbor Pharmacy 44-674
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF PM
acetaminophen, aspirin, diphenhydramine citrate tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-456 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 250 mg DIPHENHYDRAMINE CITRATE (UNII: 4OD433S209) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE CITRATE 38 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MALTODEXTRIN (UNII: 7CVR7L4A2D) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SODIUM SULFATE (UNII: 0YPR65R21J) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ZINC STEARATE (UNII: H92E6QA4FV) Product Characteristics Color blue Score no score Shape OVAL Size 17mm Flavor Imprint Code 674 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-456-71 1 in 1 CARTON 08/31/2016 1 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 08/31/2016 Labeler - Amerisource Bergen (007914906) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(46122-456) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(46122-456) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(46122-456) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(46122-456) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(46122-456)