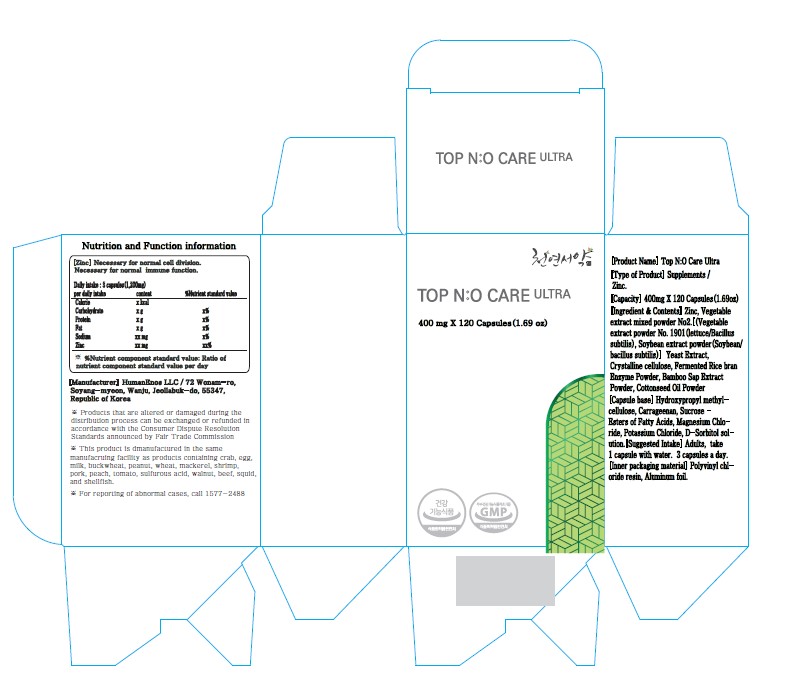
TOP NO CARE ULTRA (HELPS IMPROVE MATABOLIC DISORDER, LETTUCE FERMENTED POWDER)- nitric oxide, zinc, glutathione capsule
HumanEnos LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Top NO Care Ultra (Helps improve matabolic disorder, Lettuce fermented powder)
| TOP NO CARE ULTRA (HELPS IMPROVE MATABOLIC DISORDER, LETTUCE FERMENTED POWDER)
nitric oxide, zinc, glutathione capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - HumanEnos LLC (695801540) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HumanEnos LLC | 695801540 | manufacture(83202-9325) | |
Revised: 2/2023
Document Id: f515b5c6-dec6-6bc0-e053-2a95a90a46ab
Set id: f515b5c6-dec5-6bc0-e053-2a95a90a46ab
Version: 1
Effective Time: 20230218
HumanEnos LLC