Label: IBSIBD NO 180 START (HELPS IMPROVE INTESTINAL HEALTHY AND IMMUNITY, LETTUCE AND SOYBEAN FERMENTED POWDER)- nitric oxide, vitamin d3, glutathione capsule
- NDC Code(s): 83202-1405-1
- Packager: HumanEnos LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- These highlights do not include all the information needed to use. See full prescribing information.
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- PURPOSE
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
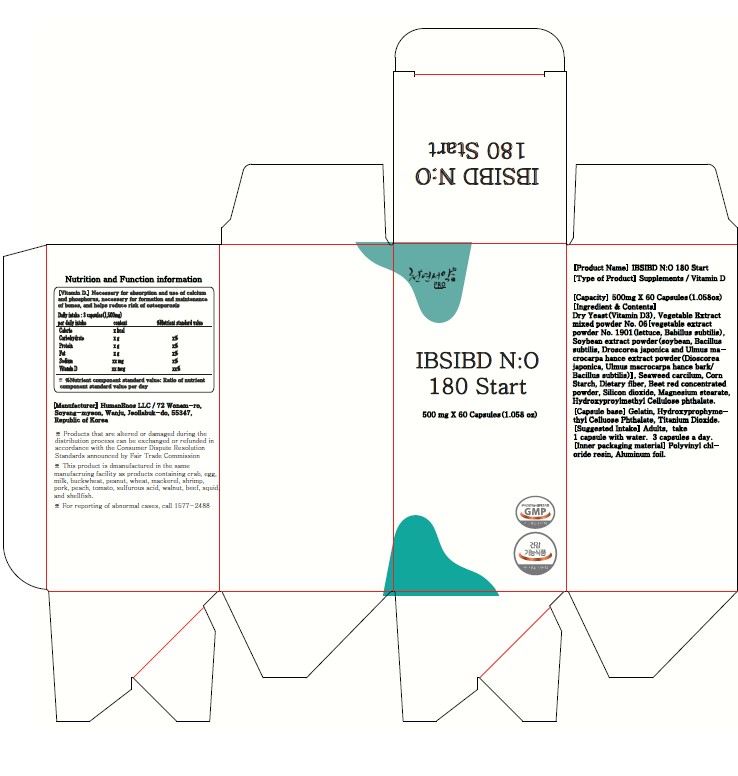
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IBSIBD NO 180 START (HELPS IMPROVE INTESTINAL HEALTHY AND IMMUNITY, LETTUCE AND SOYBEAN FERMENTED POWDER)
nitric oxide, vitamin d3, glutathione capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83202-1405 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITRIC OXIDE (UNII: 31C4KY9ESH) (NITRIC OXIDE - UNII:31C4KY9ESH) NITRIC OXIDE 500 mg in 500 mg Inactive Ingredients Ingredient Name Strength CORN STARCH, 3-E-DODECENYL SUCCINIC ANHYDRIDE MODIFIED, CALCIUM (UNII: U4XAB0853W) 1 mg in 500 mg SILICON DIOXIDE (UNII: ETJ7Z6XBU4) 1 mg in 500 mg Product Characteristics Color white Score score with uneven pieces Shape CAPSULE Size 500mm Flavor FRUIT Imprint Code capsule Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83202-1405-1 60 mg in 1 PACKAGE; Type 0: Not a Combination Product 02/13/2024 02/12/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/13/2024 02/12/2025 Labeler - HumanEnos LLC (695801540) Establishment Name Address ID/FEI Business Operations HumanEnos LLC 695801540 manufacture(83202-1405)