5VET002 VETA K-1- phytonadione capsule, liquid filled
Best Formulations Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
IMPORTANT
This is a bulk packaged product. It is not intended for use in its present form. Further processing is in accordance with all application standards and regulations. Storage conditions: 30% to 50% relative humidity at 59 degrees - 86 degrees F ( 15 degrees - 30 degrees C). Do not refrigerate.
Bulk Packaged Product Label Drug
Best Formulations 17758 Rowland Street City of Industry, CA 91748 (626) 912-9998
DESCRIPTION
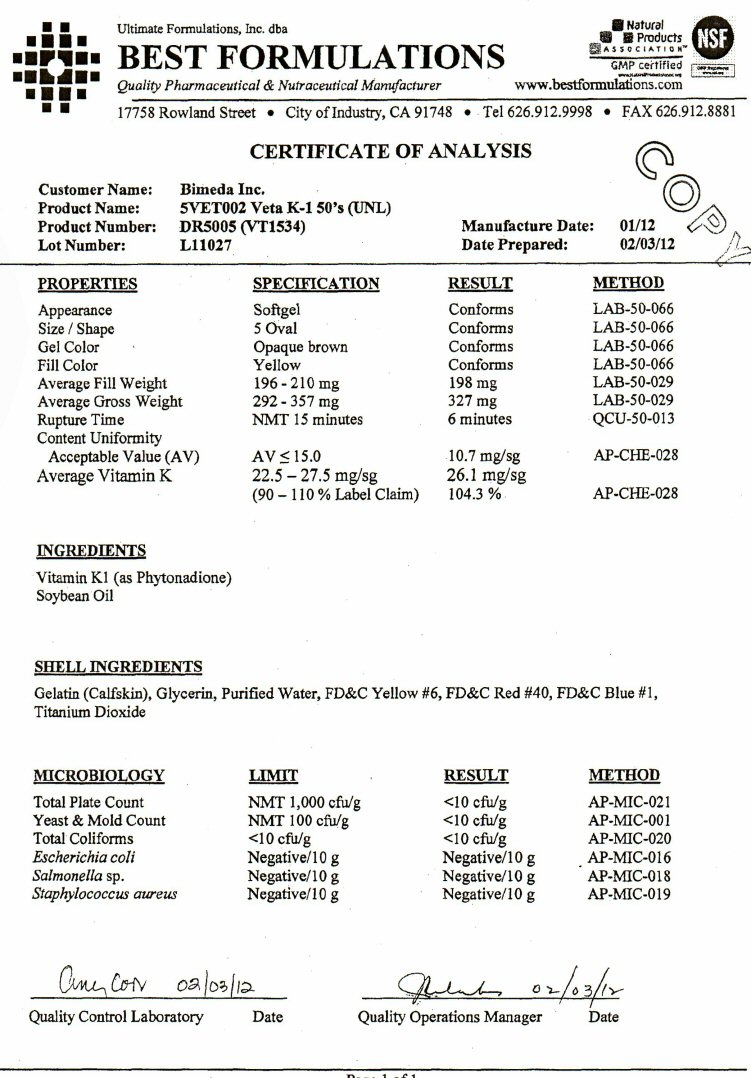
PRODUCT: VIT. K-1 GEL CAPS
CODE: DR5005
SIZE: 5 Oval
LOT NO.: L11027
NET WEIGHT: 16.33 Lbs
COUNT: 23,000 Softgels
MFG DATE: 01 - 2012
BY: M.Z.
INGREDIENTS
Vitamin K-1 Phytonadione, Soybean Oil, Gelatin, Glycerin, Purified Water, FDandC Blue # 1, Titanium Dioxide
DRUG
IMPORTANT
This is a bulk packaged product. It is not intended for use in its present form. Further processing is in accordance with all application standards and regulations. Storage conditions: 30% to 50% relative humidity at 59 degrees - 86 degrees F ( 15 degrees - 30 degrees C). Do not refrigerate.

res
| 5VET002 VETA K-1
phytonadione capsule, liquid filled |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Best Formulations Inc. (147341796) |
| Registrant - Best Formulations Inc. (147341796) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Best Formulations Inc. | 147341796 | manufacture, api manufacture | |