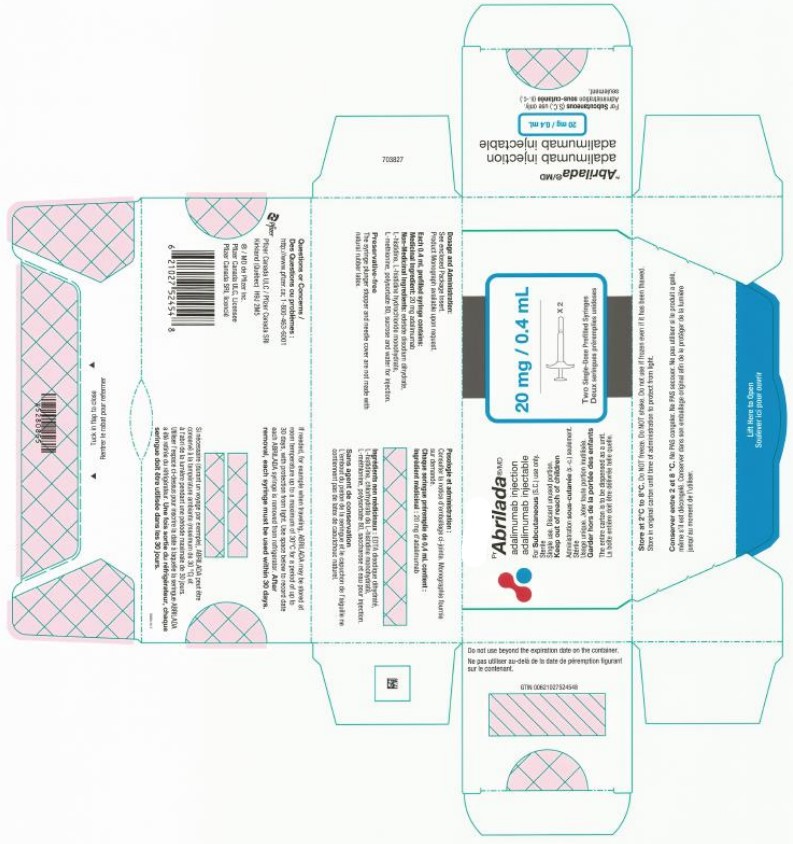
ABRILADA- adalimumab-afzb injection, solution
Catalent Indiana, LLC
----------
| ABRILADA
adalimumab-afzb injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Catalent Indiana, LLC (172209277) |
Revised: 9/2023
Document Id: 16c2517e-623a-4c5c-a714-017c71f65d5d
Set id: f2ee682a-8ea6-4068-bdbe-8a9e7d1a2514
Version: 7
Effective Time: 20230926
Catalent Indiana, LLC