Label: MIOSTAT- carbachol solution
- NDC Code(s): 0065-0023-15
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
MIOSTAT™ (carbachol intraocular solution, USP) 0.01% is a sterile balanced salt solution of carbachol for intraocular injection. The active ingredient is represented by the chemical structure:

Established Name: Carbachol
Chemical Name: Ethanaminium, 2-[(aminocarbonyl)oxy]-N,N,Ntrimethyl-, chloride.
Molecular Formula: C6H15CIN2O2
Molecular Weight: 182.65Each mL of MIOSTAT™ (carbachol intraocular solution, USP) 0.01%contains: Active: carbachol 0.01%.
Inactives: sodium chloride 0.64%, potassium chloride 0.075%, calcium chloride dehydrate 0.048%, magnesium chloride hexahydrate 0.03%, sodium acetate trihydrate 0.39%, sodium citrate dihydrate 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH) and Water for Injection. pH range is 6.5-7.5. - CLINICAL PHARMACOLOGY:
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
-
WARNINGS:
For single-dose intraocular use only. Discard unused portion. Intraocular carbachol 0.01% should be used with caution in patients with acute cardiac failure, bronchial asthma, peptic ulcer, hyperthyroidism, G.I. spasm, urinary tract obstruction and Parkinson's disease. The vial stopper contains natural rubber (latex) which may cause severe allergic reactions.
- PRECAUTIONS:
- Pediatric Use:
- Geriatric Use:
-
ADVERSE REACTIONS:
Ocular: Corneal clouding, persistent bullous keratopathy, retinal detachment and postoperative iritis following cataract extraction have been reported.
Systemic: Side effects such as flushing, sweating, epigastric distress, abdominal cramps, tightness in urinary bladder, and headache have been reported with topical or systemic application of carbachol.
The following additional reactions have been identified during post-approval use of MIOSTAT (carbachol intraocular solution, USP) 0.01% in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to MIOSTAT, or a combination of these factors, include: corneal edema, drug effect prolonged (miosis), eye inflammation, eye pain, intraocular pressure increased, ocular hyperemia, vision blurred, visual impairment, and vomiting.
-
DOSAGE AND ADMINISTRATION:
Aseptically remove the sterile vial from the blister package by peeling the backing paper and dropping the vial onto a sterile tray. Withdraw the contents into a dry sterile syringe, and replace the needle with an atraumatic cannula prior to intraocular instillation. No more than one-half milliliter should be gently instilled into the anterior chamber for the production of satisfactory miosis. It may be instilled before or after securing sutures. Miosis is usually maximal within two to five minutes after application.
- HOW SUPPLIED:
-
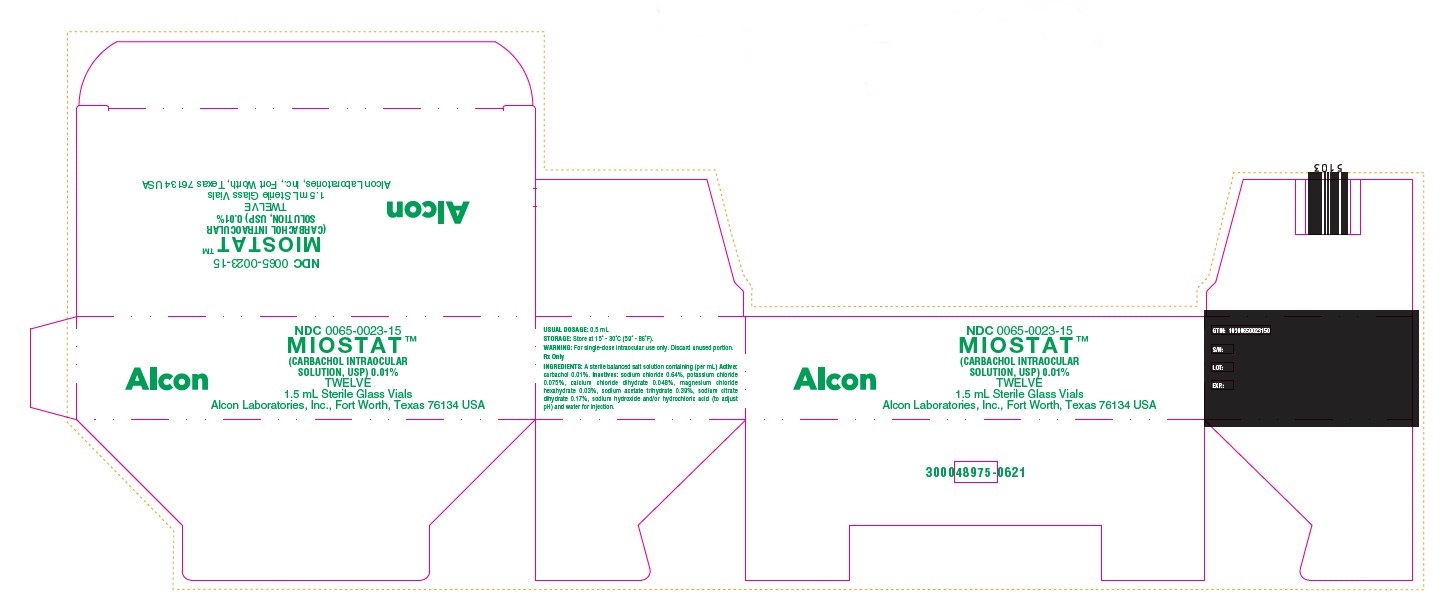
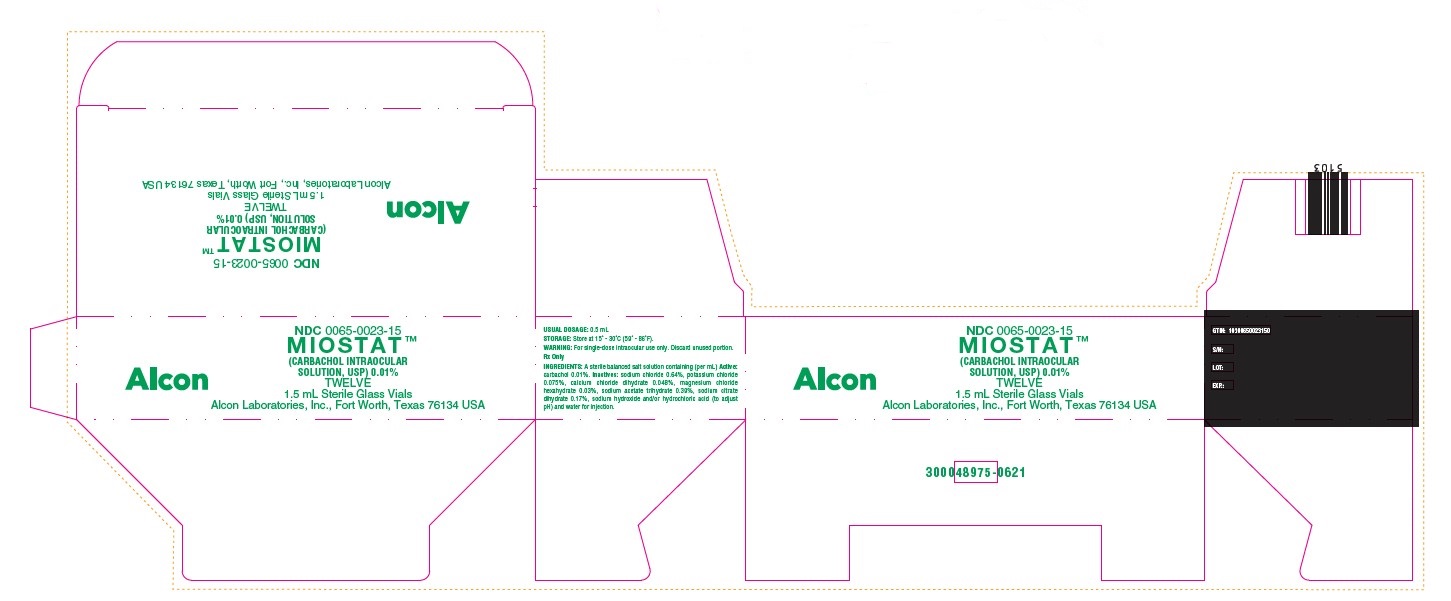
PRINCIPAL DISPLAY PANEL
NDC 0065-0023-15
MIOSTAT™
(CARBACHOL INTRAOCULAR
SOLUTION, USP) 0.01%
TWELVE
1.5 mL Sterile Glass Vials
Alcon Laboratories, Inc., Fort Worth, Texas 76134 USA
Alcon
USUAL DOSAGE: 0.5 mL
STORAGE: Store at 15 - 30C (59 - 86F).
WARNING: For single-dose intraocular use only. Discard unused portion.
Rx Only
INGREDIENTS: A sterile balanced salt solution containing (per mL) Active:
carbachol 0.01%. Inactives: sodium chloride 0.64%, potassium chloride
0.075%, calcium chloride dihydrate 0.048%, magnesium chloride
hexahydrate 0.03%, sodium acetate trihydrate 0.39%, sodium citrate
dihydrate 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust
pH) and water for injection.
GTIN: 10300650023150
S/N:
LOT:
EXP.:
300048975-0621
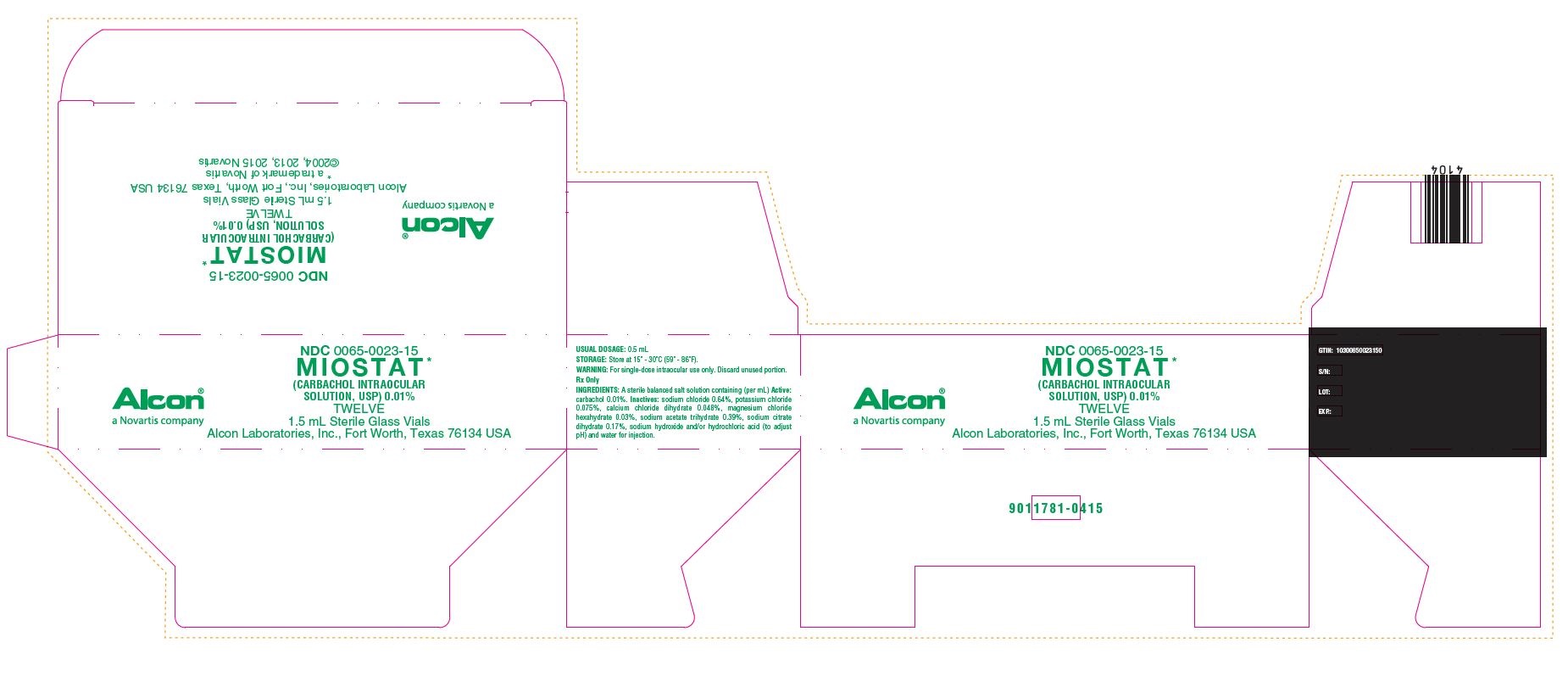
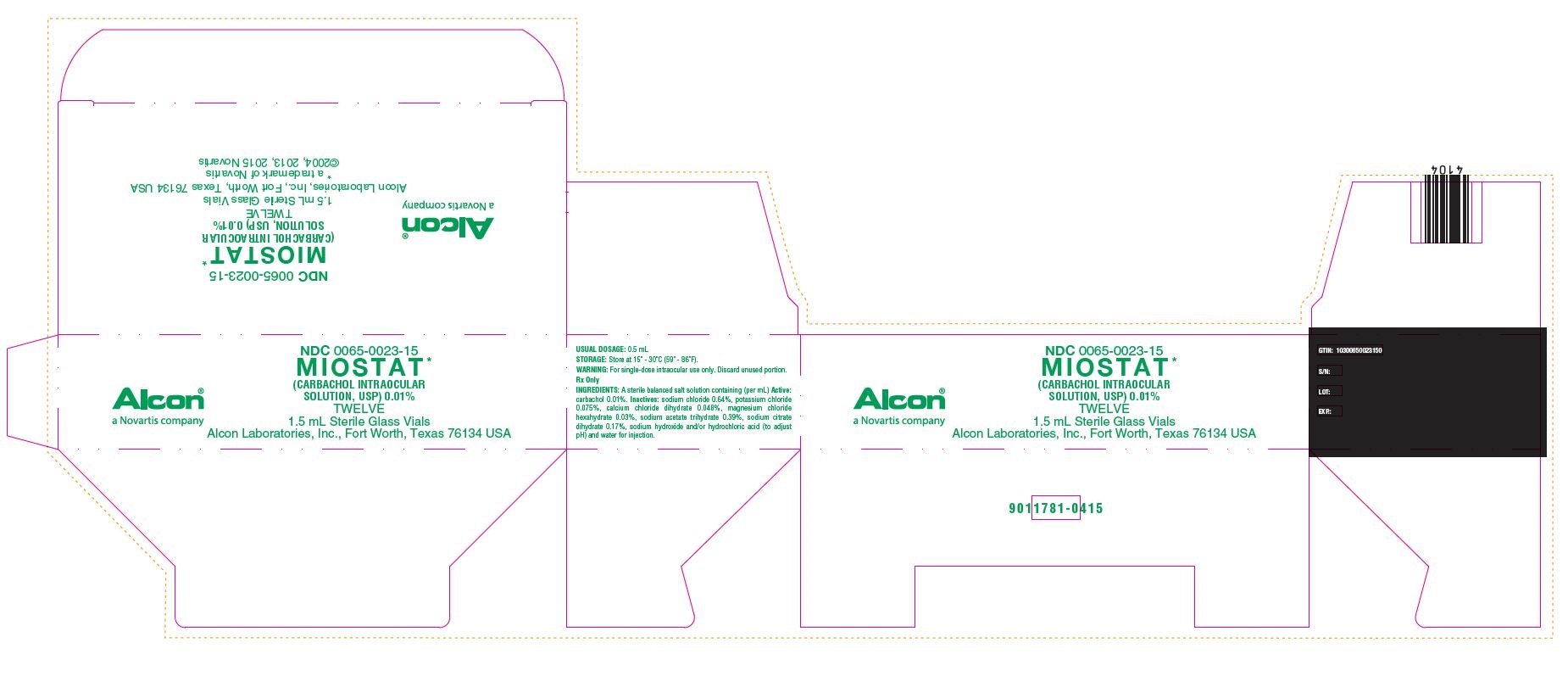
NDC 0065-0023-15
MIOSTAT*
(CARBACHOL INTRAOCULAR SOLUTION, USP) 0.01%
TWELVE
1.5 mL Sterile Glass Vials
Alcon Laboratories, Inc., Fort Worth, Texas 76134 USA
* a trademark of Novartis
©2004, 2013, 2015 Novartis
Alcon®
a Novartis company
USUAL DOSAGE: 0.5 mL
STORAGE: Store at 15 - 30C (59 - 86F).
Rx Only
INGREDIENTS: A sterile balanced salt solution containing (per mL) Active: carbachol 0.01%. Inactives: sodium chloride 0.64%, potassium chloride 0.075%, calcium chloride dihydrate 0.048%, magnesium chloride hexahydrate 0.03%, sodium acetate trihydrate 0.39%, sodium citrate dihydrate 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH) and water for injection.
GTIN: 10300650023150
S/N
LOT:
EXP.:
9011781-0415
MIOSTAT™
(carbachol intraocular solution, USP) 0.01%
Alcon
300056865
LOT: EXP.:

MIOSTAT®
(carbachol intraocular solution, USP) 0.01%
Alcon®
© 2001, 2018 Alcon, Inc.
H15348-0718
LOT: EXP.:

LOT: EXP: 305190-1104
NDC 0065-0023-15
MIOSTAT®
(CARBACHOL INTRAOCULAR SOLUTION, USP) 0.01% 1.5mL
Rx Only. Sterile Unless Package Open or Damaged Read enclosed insert. INGREDIENTS: Active: carbachol 0.01%. Inactives: sodium chloride 0.64%, potassium chloride 0.075%, calcium chloride dihydrate 0.048%, magnesium chloride hexahydrate 0.03%, sodium acetate trihydrate 0.39%, sodium citrate dihydrate 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH) and water for injection. USUAL DOSAGE: 0.5mL STORAGE: Store at 15° - 30°C (59° - 86°F).
©2004 Alcon, Inc. Alcon Labs., Inc. Fort Worth, TX. 76134

-
INGREDIENTS AND APPEARANCE
MIOSTAT
carbachol solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0065-0023 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARBACHOL (UNII: 8Y164V895Y) (Carbamoylcholine - UNII:54Z8M50D6Q) CARBACHOL 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) SODIUM ACETATE (UNII: 4550K0SC9B) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-0023-15 12 in 1 CARTON 04/15/1974 1 1.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA016968 04/15/1974 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0023) Establishment Name Address ID/FEI Business Operations Siegfried AG 482824026 api manufacture(0065-0023)