GAS RELIEF INFANTS- simethicone emulsion
INFIRST HEALTHCARE
----------
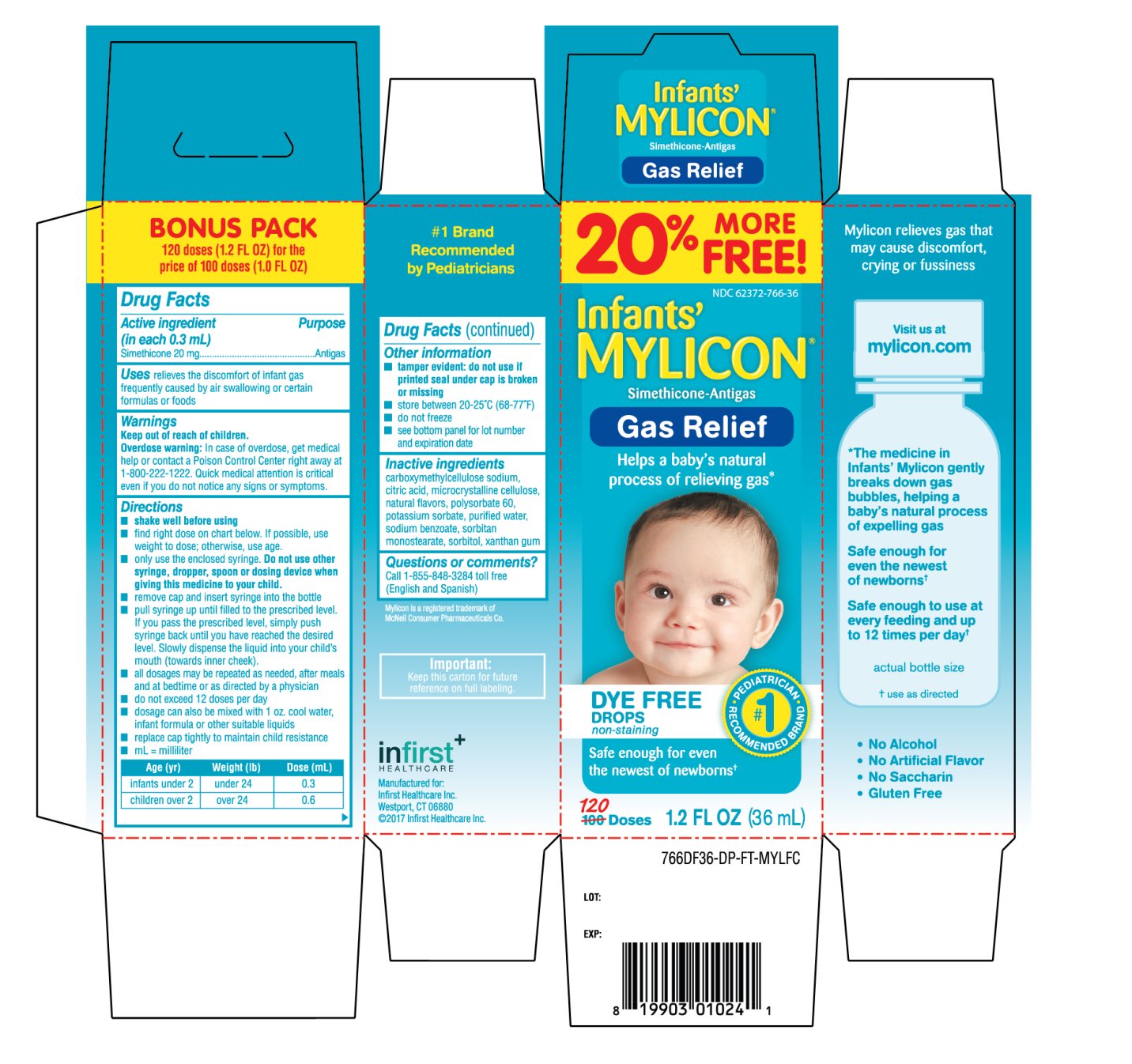
INFIRST Gas Relief Infants’ Drops Drug Facts
Uses
relieves the discomfort of infant gas frequently caused by air swallowing or certain formulas or foods
Directions
- shake well before using
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- only use the enclosed syringe. Do not use other syringe, dropper, spoon or dosing device when giving this medicine to your child.
- remove cap and insert syringe into the bottle
- pull syringe up until filled to the prescribed level. If you pass the prescribed level, simply push syringe back until you have reached the desired level. Slowly dispense the liquid into your child’s mouth (towards inner cheek).
- all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician
- do not exceed 12 doses per day
- dosage can also be mixed with 1 oz. cool water, infant formula or other suitable liquids
- replace cap tightly to maintain child resistance
- mL = milliliter
|
Age (yr) |
Weight (lb) |
Dose (mL) |
|
infants under 2 |
under 24 |
0.3 |
|
children over 2 |
over 24 |
0.6 |
Other information
- tamper evident: do not use if printed bottle wrap is broken or missing
- store between 20-25°C (68-77°F)
- do not freeze
- see bottom panel for lot number and expiration date
Inactive ingredients
Carboxymethylcellulose sodium, citric acid, microcrystalline cellulose, natural flavors, polysorbate 60, potassium sorbate, purified water, sodium benzoate, sorbitan monostearate, sorbitol, xanthan gum
Principal Display Panel
20% MORE FREE!
62372-766-36
Infants’ MYLICON®
Simethicone -Antigas
Gas Relief
Helps a baby’s natural process of relieving gas*
DYE FREE DROPS
Non-staining
Anti-Gas
PEDIATRICIAN RECOMMENDED #1 PEDIATRICIAN
Safe enough for even the newest of newborn
120 Doses
1.2 FL OZ (36 mL)
Mylicon is a registered trademark of McNeil Consumer Pharmaceuticals Co.
Important: Keep this carton for future reference on full labeling.
infirst†
HEALTHCARE
Manufactured for: Infirst Healthcare Inc. Westport, CT 06880@2018 Infirst Healthcare Inc.
#1 Brand Recommended by Pediatricians
BONUS PACK
120doses (1.2 FL OZ) for the price of 100 doses (1.0 FL OZ)
Mylicon relives gas that may cause discomfort, crying or fussiness
Visit us at Mylicon.com
* The medicine in Infants’ Mylicon gently breaks down gas bubbles, helping a baby’s natural process of expelling gas
Safe enough for even the newest of newborns*
Safe enough to use at every feeding and up to 12 times per day*
Actual bottle size
* use as directed
- • No Alcohal
- • No Artificial Flavor
- • No Saccharin
- • Gluten Free
| GAS RELIEF
INFANTS
simethicone emulsion |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - INFIRST HEALTHCARE (079159739) |