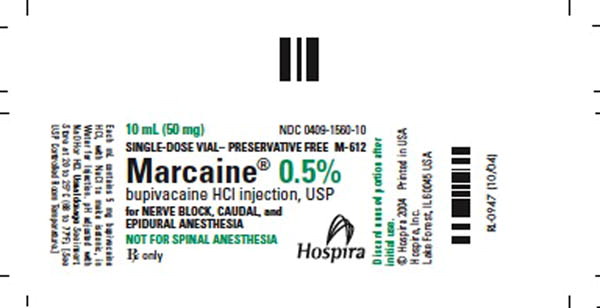
Label: PHYSICIANS EZ USE M-PRED- methylprednisolone acetate, bupivacaine hydrochloride, povidone-iodine kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 0409-1560-10, 0703-0043-01, 10819-3883-3, 63187-352-01 - Packager: Proficient Rx LP
- This is a repackaged label.
- Source NDC Code(s): 76420-520
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 30, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
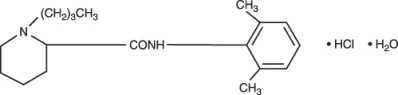
Bupivacaine hydrochloride is 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, monohydrochloride, monohydrate, a white crystalline powder that is freely soluble in 95 percent ethanol, soluble in water, and slightly soluble in chloroform or acetone. It has the following structural formula:
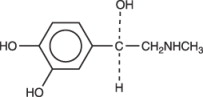
Epinephrine is (-)-3,4-Dihydroxy-α-[(methylamino)methyl] benzyl alcohol. It has the following structural formula:
MARCAINE is available in sterile isotonic solutions with and without epinephrine (as bitartrate) 1:200,000 for injection via local infiltration, peripheral nerve block, and caudal and lumbar epidural blocks. Solutions of MARCAINE may be autoclaved if they do not contain epinephrine. Solutions are clear and colorless.
Bupivacaine is related chemically and pharmacologically to the aminoacyl local anesthetics. It is a homologue of mepivacaine and is chemically related to lidocaine. All three of these anesthetics contain an amide linkage between the aromatic nucleus and the amino, or piperidine group. They differ in this respect from the procaine-type local anesthetics, which have an ester linkage.
MARCAINE — Sterile isotonic solutions containing sodium chloride. In multiple-dose vials, each mL also contains 1 mg methylparaben as antiseptic preservative. The pH of these solutions is adjusted to between 4 and 6.5 with sodium hydroxide or hydrochloric acid.
MARCAINE with epinephrine 1:200,000 (as bitartrate)—Sterile isotonic solutions containing sodium chloride. Each mL contains bupivacaine hydrochloride and 0.0091 mg epinephrine bitartrate, with 0.5 mg sodium metabisulfite, 0.001 mL monothioglycerol, and 2 mg ascorbic acid as antioxidants, 0.0017 mL 60% sodium lactate buffer, and 0.1 mg edetate calcium disodium as stabilizer. In multiple-dose vials, each mL also contains 1 mg methylparaben as antiseptic preservative. The pH of these solutions is adjusted to between 3.4 and 4.5 with sodium hydroxide or hydrochloric acid. The specific gravity of MARCAINE 0.5% with epinephrine 1:200,000 (as bitartrate) at 25°C is 1.008 and at 37°C is 1.008.
-
CLINICAL PHARMACOLOGY
Local anesthetics block the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination, and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
Systemic absorption of local anesthetics produces effects on the cardiovascular and central nervous systems (CNS). At blood concentrations achieved with normal therapeutic doses, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations depress cardiac conduction and excitability, which may lead to atrioventricular block, ventricular arrhythmias, and cardiac arrest, sometimes resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. Recent clinical reports and animal research suggest that these cardiovascular changes are more likely to occur after unintended intravascular injection of bupivacaine. Therefore, incremental dosing is necessary.
Following systemic absorption, local anesthetics can produce central nervous system stimulation, depression, or both. Apparent central stimulation is manifested as restlessness, tremors and shivering progressing to convulsions, followed by depression and coma progressing ultimately to respiratory arrest. However, the local anesthetics have a primary depressant effect on the medulla and on higher centers. The depressed stage may occur without a prior excited state.
Pharmacokinetics: The rate of systemic absorption of local anesthetics is dependent upon the total dose and concentration of drug administered, the route of administration, the vascularity of the administration site, and the presence or absence of epinephrine in the anesthetic solution. A dilute concentration of epinephrine (1:200,000 or 5 mcg/mL) usually reduces the rate of absorption and peak plasma concentration of MARCAINE, permitting the use of moderately larger total doses and sometimes prolonging the duration of action.
The onset of action with MARCAINE is rapid and anesthesia is long lasting. The duration of anesthesia is significantly longer with MARCAINE than with any other commonly used local anesthetic. It has also been noted that there is a period of analgesia that persists after the return of sensation, during which time the need for strong analgesics is reduced.
The onset of action following dental injections is usually 2 to 10 minutes and anesthesia may last two or three times longer than lidocaine and mepivacaine for dental use, in many patients up to 7 hours. The duration of anesthetic effect is prolonged by the addition of epinephrine 1:200,000.
Local anesthetics are bound to plasma proteins in varying degrees. Generally, the lower the plasma concentration of drug the higher the percentage of drug bound to plasma proteins.
Local anesthetics appear to cross the placenta by passive diffusion. The rate and degree of diffusion is governed by (1) the degree of plasma protein binding, (2) the degree of ionization, and (3) the degree of lipid solubility. Fetal/ maternal ratios of local anesthetics appear to be inversely related to the degree of plasma protein binding, because only the free, unbound drug is available for placental transfer. MARCAINE with a high protein binding capacity (95%) has a low fetal/maternal ratio (0.2 to 0.4). The extent of placental transfer is also determined by the degree of ionization and lipid solubility of the drug. Lipid soluble, nonionized drugs readily enter the fetal blood from the maternal circulation.
Depending upon the route of administration, local anesthetics are distributed to some extent to all body tissues, with high concentrations found in highly perfused organs such as the liver, lungs, heart, and brain.
Pharmacokinetic studies on the plasma profile of MARCAINE after direct intravenous injection suggest a three-compartment open model. The first compartment is represented by the rapid intravascular distribution of the drug. The second compartment represents the equilibration of the drug throughout the highly perfused organs such as the brain, myocardium, lungs, kidneys, and liver. The third compartment represents an equilibration of the drug with poorly perfused tissues, such as muscle and fat. The elimination of drug from tissue distribution depends largely upon the ability of binding sites in the circulation to carry it to the liver where it is metabolized.
After injection of MARCAINE for caudal, epidural, or peripheral nerve block in man, peak levels of bupivacaine in the blood are reached in 30 to 45 minutes, followed by a decline to insignificant levels during the next three to six hours.
Various pharmacokinetic parameters of the local anesthetics can be significantly altered by the presence of hepatic or renal disease, addition of epinephrine, factors affecting urinary pH, renal blood flow, the route of drug administration, and the age of the patient. The half-life of MARCAINE in adults is 2.7 hours and in neonates 8.1 hours.
In clinical studies, elderly patients reached the maximal spread of analgesia and maximal motor blockade more rapidly than younger patients. Elderly patients also exhibited higher peak plasma concentrations following administration of this product. The total plasma clearance was decreased in these patients.
Amide-type local anesthetics such as MARCAINE are metabolized primarily in the liver via conjugation with glucuronic acid. Patients with hepatic disease, especially those with severe hepatic disease, may be more susceptible to the potential toxicities of the amide-type local anesthetics. Pipecoloxylidine is the major metabolite of MARCAINE.
The kidney is the main excretory organ for most local anesthetics and their metabolites. Urinary excretion is affected by urinary perfusion and factors affecting urinary pH. Only 6% of bupivacaine is excreted unchanged in the urine.
When administered in recommended doses and concentrations, MARCAINE does not ordinarily produce irritation or tissue damage and does not cause methemoglobinemia.
-
INDICATIONS AND USAGE
MARCAINE is indicated for the production of local or regional anesthesia or analgesia for surgery, dental and oral surgery procedures, diagnostic and therapeutic procedures, and for obstetrical procedures. Only the 0.25% and 0.5% concentrations are indicated for obstetrical anesthesia. (See WARNINGS.)
Experience with nonobstetrical surgical procedures in pregnant patients is not sufficient to recommend use of 0.75% concentration of MARCAINE in these patients.
MARCAINE is not recommended for intravenous regional anesthesia (Bier Block). See WARNINGS.
The routes of administration and indicated MARCAINE concentrations are:
∙ local infiltration 0.25%
∙ peripheral nerve block 0.25% and 0.5%
∙ retrobulbar block 0.75%
∙ sympathetic block 0.25%
∙ lumbar epidural 0.25%, 0.5%, and 0.75%
(0.75% not for obstetrical anesthesia)
∙ caudal 0.25% and 0.5%
∙ epidural test dose 0.5% with epinephrine 1:200,000
∙ dental blocks 0.5% with epinephrine 1:200,000
(See DOSAGE AND ADMINISTRATION for additional information.)
Standard textbooks should be consulted to determine the accepted procedures and techniques for the administration of MARCAINE.
-
CONTRAINDICATIONS
MARCAINE is contraindicated in obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death.
MARCAINE is contraindicated in patients with a known hypersensitivity to it or to any local anesthetic agent of the amide-type or to other components of MARCAINE solutions.
-
WARNINGS
THE 0.75% CONCENTRATION OF MARCAINE IS NOT RECOMMENDED FOR OBSTETRICAL ANESTHESIA. THERE HAVE BEEN REPORTS OF CARDIAC ARREST WITH DIFFICULT RESUSCITATION OR DEATH DURING USE OF MARCAINE FOR EPIDURAL ANESTHESIA IN OBSTETRICAL PATIENTS. IN MOST CASES, THIS HAS FOLLOWED USE OF THE 0.75% CONCENTRATION. RESUSCITATION HAS BEEN DIFFICULT OR IMPOSSIBLE DESPITE APPARENTLY ADEQUATE PREPARATION AND APPROPRIATE MANAGEMENT. CARDIAC ARREST HAS OCCURRED AFTER CONVULSIONS RESULTING FROM SYSTEMIC TOXICITY, PRESUMABLY FOLLOWING UNINTENTIONAL INTRAVASCULAR INJECTION. THE 0.75% CONCENTRATION SHOULD BE RESERVED FOR SURGICAL PROCEDURES WHERE A HIGH DEGREE OF MUSCLE RELAXATION AND PROLONGED EFFECT ARE NECESSARY.
LOCAL ANESTHETICS SHOULD ONLY BE EMPLOYED BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF DOSE-RELATED TOXICITY AND OTHER ACUTE EMERGENCIES WHICH MIGHT ARISE FROM THE BLOCK TO BE EMPLOYED, AND THEN ONLY AFTER INSURING THE IMMEDIATE AVAILABILITY OF OXYGEN, OTHER RESUSCITATIVE DRUGS, CARDIOPULMONARY RESUSCITATIVE EQUIPMENT, AND THE PERSONNEL RESOURCES NEEDED FOR PROPER MANAGEMENT OF TOXIC REACTIONS AND RELATED EMERGENCIES. (See also ADVERSE REACTIONS, PRECAUTIONS, and OVERDOSAGE.) DELAY IN PROPER MANAGEMENT OF DOSE-RELATED TOXICITY, UNDERVENTILATION FROM ANY CAUSE, AND/OR ALTERED SENSITIVITY MAY LEAD TO THE DEVELOPMENT OF ACIDOSIS, CARDIAC ARREST AND, POSSIBLY, DEATH.
Local anesthetic solutions containing antimicrobial preservatives, i.e., those supplied in multiple-dose vials, should not be used for epidural or caudal anesthesia because safety has not been established with regard to intrathecal injection, either intentionally or unintentionally, of such preservatives.
Intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are not associated with these findings. The time of onset of symptoms, such as joint pain, stiffness and loss of motion can be variable, but may begin as early as the 2nd month after surgery. Currently, there is no effective treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
It is essential that aspiration for blood or cerebrospinal fluid (where applicable) be done prior to injecting any local anesthetic, both the original dose and all subsequent doses, to avoid intravascular or subarachnoid injection. However, a negative aspiration does not ensure against an intravascular or subarachnoid injection.
MARCAINE with epinephrine 1:200,000 or other vasopressors should not be used concomitantly with ergot-type oxytocic drugs, because a severe persistent hypertension may occur. Likewise, solutions of MARCAINE containing a vasoconstrictor, such as epinephrine, should be used with extreme caution in patients receiving monoamineoxidase inhibitors (MAOI) or antidepressants of the triptyline or imipramine types, because severe prolonged hypertension may result.
Until further experience is gained in pediatric patients younger than 12 years, administration of MARCAINE in this age group is not recommended.
Mixing or the prior or intercurrent use of any other local anesthetic with MARCAINE cannot be recommended because of insufficient data on the clinical use of such mixtures.
There have been reports of cardiac arrest and death during the use of MARCAINE for intravenous regional anesthesia (Bier Block). Information on safe dosages and techniques of administration of MARCAINE in this procedure is lacking. Therefore, MARCAINE is not recommended for use in this technique.
MARCAINE with epinephrine 1:200,000 contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people. Single-dose ampuls and single-dose vials of MARCAINE without epinephrine do not contain sodium metabisulfite.
-
PRECAUTIONS
General: The safety and effectiveness of local anesthetics depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use. (See WARNINGS, ADVERSE REACTIONS, and OVERDOSAGE.) During major regional nerve blocks, the patient should have IV fluids running via an indwelling catheter to assure a functioning intravenous pathway. The lowest dosage of local anesthetic that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. The rapid injection of a large volume of local anesthetic solution should be avoided and fractional (incremental) doses should be used when feasible.
Epidural Anesthesia: During epidural administration of MARCAINE, 0.5% and 0.75% solutions should be administered in incremental doses of 3 mL to 5 mL with sufficient time between doses to detect toxic manifestations of unintentional intravascular or intrathecal injection. Injections should be made slowly, with frequent aspirations before and during the injection to avoid intravascular injection. Syringe aspirations should also be performed before and during each supplemental injection in continuous (intermittent) catheter techniques. An intravascular injection is still possible even if aspirations for blood are negative.
During the administration of epidural anesthesia, it is recommended that a test dose be administered initially and the effects monitored before the full dose is given. When using a “continuous” catheter technique, test doses should be given prior to both the original and all reinforcing doses, because plastic tubing in the epidural space can migrate into a blood vessel or through the dura. When clinical conditions permit, the test dose should contain epinephrine (10 mcg to 15 mcg has been suggested) to serve as a warning of unintended intravascular injection. If injected into a blood vessel, this amount of epinephrine is likely to produce a transient “epinephrine response” within 45 seconds, consisting of an increase in heart rate and/or systolic blood pressure, circumoral pallor, palpitations, and nervousness in the unsedated patient. The sedated patient may exhibit only a pulse rate increase of 20 or more beats per minute for 15 or more seconds. Therefore, following the test dose, the heart rate should be monitored for a heart rate increase. Patients on beta-blockers may not manifest changes in heart rate, but blood pressure monitoring can detect a transient rise in systolic blood pressure. The test dose should also contain 10 mg to 15 mg of MARCAINE or an equivalent amount of another local anesthetic to detect an unintended intrathecal administration. This will be evidenced within a few minutes by signs of spinal block (e.g., decreased sensation of the buttocks, paresis of the legs, or, in the sedated patient, absent knee jerk). The Test Dose formulation of MARCAINE contains 15 mg of bupivacaine and 15 mcg of epinephrine in a volume of 3 mL. An intravascular or subarachnoid injection is still possible even if results of the test dose are negative. The test dose itself may produce a systemic toxic reaction, high spinal or epinephrine-induced cardiovascular effects.
Injection of repeated doses of local anesthetics may cause significant increases in plasma levels with each repeated dose due to slow accumulation of the drug or its metabolites, or to slow metabolic degradation. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients and acutely ill patients should be given reduced doses commensurate with their age and physical status. Local anesthetics should also be used with caution in patients with hypotension or heartblock.
Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient’s state of consciousness should be performed after each local anesthetic injection. It should be kept in mind at such times that restlessness, anxiety, incoherent speech, lightheadedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, depression, or drowsiness may be early warning signs of central nervous system toxicity.
Local anesthetic solutions containing a vasoconstrictor should be used cautiously and in carefully restricted quantities in areas of the body supplied by end arteries or having otherwise compromised blood supply such as digits, nose, external ear, or penis. Patients with hypertensive vascular disease may exhibit exaggerated vasoconstrictor response. Ischemic injury or necrosis may result.
Because amide-local anesthetics such as MARCAINE are metabolized by the liver, these drugs, especially repeat doses, should be used cautiously in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations. Local anesthetics should also be used with caution in patients with impaired cardiovascular function because they may be less able to compensate for functional changes associated with the prolongation of AV conduction produced by these drugs.
Serious dose-related cardiac arrhythmias may occur if preparations containing a vasoconstrictor such as epinephrine are employed in patients during or following the administration of potent inhalation anesthetics. In deciding whether to use these products concurrently in the same patient, the combined action of both agents upon the myocardium, the concentration and volume of vasoconstrictor used, and the time since injection, when applicable, should be taken into account.
Many drugs used during the conduct of anesthesia are considered potential triggering agents for familial malignant hyperthermia. Because it is not known whether amide-type local anesthetics may trigger this reaction and because the need for supplemental general anesthesia cannot be predicted in advance, it is suggested that a standard protocol for management should be available. Early unexplained signs of tachycardia, tachypnea, labile blood pressure, and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the suspect triggering agent(s) and prompt institution of treatment, including oxygen therapy, indicated supportive measures and dantrolene. (Consult dantrolene sodium intravenous package insert before using.)
Use in Head and Neck Area: Small doses of local anesthetics injected into the head and neck area, including retrobulbar, dental, and stellate ganglion blocks, may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. The injection procedures require the utmost care. Confusion, convulsions, respiratory depression, and/or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. They may also be due to puncture of the dural sheath of the optic nerve during retrobulbar block with diffusion of any local anesthetic along the subdural space to the midbrain. Patients receiving these blocks should have their circulation and respiration monitored and be constantly observed. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded. (See DOSAGE AND ADMINISTRATION.)
Use in Ophthalmic Surgery: Clinicians who perform retrobulbar blocks should be aware that there have been reports of respiratory arrest following local anesthetic injection. Prior to retrobulbar block, as with all other regional procedures, the immediate availability of equipment, drugs, and personnel to manage respiratory arrest or depression, convulsions, and cardiac stimulation or depression should be assured (see also WARNINGS and Use In Head and Neck Area, above). As with other anesthetic procedures, patients should be constantly monitored following ophthalmic blocks for signs of these adverse reactions, which may occur following relatively low total doses.
A concentration of 0.75% bupivacaine is indicated for retrobulbar block; however, this concentration is not indicated for any other peripheral nerve block, including the facial nerve, and not indicated for local infiltration, including the conjunctiva (see INDICATIONS AND USAGE and PRECAUTIONS, General). Mixing MARCAINE with other local anesthetics is not recommended because of insufficient data on the clinical use of such mixtures.
When MARCAINE 0.75% is used for retrobulbar block, complete corneal anesthesia usually precedes onset of clinically acceptable external ocular muscle akinesia. Therefore, presence of akinesia rather than anesthesia alone should determine readiness of the patient for surgery.
Use in Dentistry: Because of the long duration of anesthesia, when MARCAINE 0.5% with epinephrine is used for dental injections, patients should be cautioned about the possibility of inadvertent trauma to tongue, lips, and buccal mucosa and advised not to chew solid foods or test the anesthetized area by biting or probing.
Information for Patients: When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity, usually in the lower half of the body, following proper administration of caudal or epidural anesthesia. Also, when appropriate, the physician should discuss other information including adverse reactions in the package insert of MARCAINE.
Patients receiving dental injections of MARCAINE should be cautioned not to chew solid foods or test the anesthetized area by biting or probing until anesthesia has worn off (up to 7 hours).
Clinically Significant Drug Interactions: The administration of local anesthetic solutions containing epinephrine or norepinephrine to patients receiving monoamine oxidase inhibitors or tricyclic antidepressants may produce severe, prolonged hypertension. Concurrent use of these agents should generally be avoided. In situations when concurrent therapy is necessary, careful patient monitoring is essential.
Concurrent administration of vasopressor drugs and of ergot-type oxytocic drugs may cause severe, persistent hypertension or cerebrovascular accidents.
Phenothiazines and butyrophenones may reduce or reverse the pressor effect of epinephrine.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term studies in animals to evaluate the carcinogenic potential of bupivacaine hydrochloride have not been conducted. The mutagenic potential and the effect on fertility of bupivacaine hydrochloride have not been determined.
Pregnancy Category C: There are no adequate and well-controlled studies in pregnant women. MARCAINE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Bupivacaine hydrochloride produced developmental toxicity when administered subcutaneously to pregnant rats and rabbits at clinically relevant doses. This does not exclude the use of MARCAINE at term for obstetrical anesthesia or analgesia. (See Labor and Delivery)
Bupivacaine hydrochloride was administered subcutaneously to rats at doses of 4.4, 13.3, & 40 mg/kg and to rabbits at doses of 1.3, 5.8, & 22.2 mg/kg during the period of organogenesis (implantation to closure of the hard palate). The high doses are comparable to the daily maximum recommended human dose (MRHD) of 400 mg/day on a mg/m2 body surface area (BSA) basis. No embryo-fetal effects were observed in rats at the high dose which caused increased maternal lethality. An increase in embryo-fetal deaths was observed in rabbits at the high dose in the absence of maternal toxicity with the fetal No Observed Adverse Effect Level representing approximately 1/5th the MRHD on a BSA basis.
In a rat pre- and post-natal development study (dosing from implantation through weaning) conducted at subcutaneous doses of 4.4, 13.3, & 40 mg/kg mg/kg/day, decreased pup survival was observed at the high dose. The high dose is comparable to the daily MRHD of 400 mg/day on a BSA basis.
Labor and Delivery: SEE BOXED WARNING REGARDING OBSTETRlCAL USE OF 0.75% MARCAINE.
MARCAINE is contraindicated for obstetrical paracervical block anesthesia.
Local anesthetics rapidly cross the placenta, and when used for epidural, caudal, or pudendal block anesthesia, can cause varying degrees of maternal, fetal, and neonatal toxicity. (See CLINICAL PHARMACOLOGY, Pharmacokinetics.) The incidence and degree of toxicity depend upon the procedure performed, the type, and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus, and neonate involve alterations of the central nervous system, peripheral vascular tone, and cardiac function.
Maternal hypotension has resulted from regional anesthesia. Local anesthetics produce vasodilation by blocking sympathetic nerves. Elevating the patient’s legs and positioning her on her left side will help prevent decreases in blood pressure. The fetal heart rate also should be monitored continuously and electronic fetal monitoring is highly advisable.
Epidural, caudal, or pudendal anesthesia may alter the forces of parturition through changes in uterine contractility or maternal expulsive efforts. Epidural anesthesia has been reported to prolong the second stage of labor by removing the parturient’s reflex urge to bear down or by interfering with motor function. The use of obstetrical anesthesia may increase the need for forceps assistance.
The use of some local anesthetic drug products during labor and delivery may be followed by diminished muscle strength and tone for the first day or two of life. This has not been reported with bupivacaine.
It is extremely important to avoid aortocaval compression by the gravid uterus during administration of regional block to parturients. To do this, the patient must be maintained in the left lateral decubitus position or a blanket roll or sandbag may be placed beneath the right hip and gravid uterus displaced to the left.
Nursing Mothers: Bupivacaine has been reported to be excreted in human milk suggesting that the nursing infant could be theoretically exposed to a dose of the drug. Because of the potential for serious adverse reactions in nursing infants from bupivacaine, a decision should be made whether to discontinue nursing or not administer bupivacaine, taking into account the importance of the drug to the mother.
Pediatric Use: Until further experience is gained in pediatric patients younger than 12 years, administration of MARCAINE in this age group is not recommended. Continuous infusions of bupivacaine in children have been reported to result in high systemic levels of bupivacaine and seizures; high plasma levels may also be associated with cardiovascular abnormalities. (See WARNINGS, PRECAUTIONS, and OVERDOSAGE.)
Geriatric Use: Patients over 65 years, particularly those with hypertension, may be at increased risk for developing hypotension while undergoing anesthesia with MARCAINE. (See ADVERSE REACTIONS.)
Elderly patients may require lower doses of MARCAINE. (See PRECAUTIONS, Epidural Anesthesia and DOSAGE AND ADMINISTRATION.)
In clinical studies, differences in various pharmacokinetic parameters have been observed between elderly and younger patients. (See CLINICAL PHARMACOLOGY.)
This product is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. (See CLINICAL PHARMACOLOGY.)
-
ADVERSE REACTIONS
Reactions to MARCAINE are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs is excessive plasma levels, which may be due to overdosage, unintentional intravascular injection, or slow metabolic degradation.
The most commonly encountered acute adverse experiences which demand immediate counter-measures are related to the central nervous system and the cardiovascular system. These adverse experiences are generally dose related and due to high plasma levels which may result from overdosage, rapid absorption from the injection site, diminished tolerance, or from unintentional intravascular injection of the local anesthetic solution. In addition to systemic dose-related toxicity, unintentional subarachnoid injection of drug during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column (especially in the head and neck region) may result in underventilation or apnea (“Total or High Spinal”). Also, hypotension due to loss of sympathetic tone and respiratory paralysis or underventilation due to cephalad extension of the motor level of anesthesia may occur. This may lead to secondary cardiac arrest if untreated. Patients over 65 years, particularly those with hypertension, may be at increased risk for experiencing the hypotensive effects of MARCAINE. Factors influencing plasma protein binding, such as acidosis, systemic diseases which alter protein production, or competition of other drugs for protein binding sites, may diminish individual tolerance.
Central Nervous System Reactions: These are characterized by excitation and/or depression. Restlessness, anxiety, dizziness, tinnitus, blurred vision, or tremors may occur, possibly proceeding to convulsions. However, excitement may be transient or absent, with depression being the first manifestation of an adverse reaction. This may quickly be followed by drowsiness merging into unconsciousness and respiratory arrest. Other central nervous system effects may be nausea, vomiting, chills, and constriction of the pupils.
The incidence of convulsions associated with the use of local anesthetics varies with the procedure used and the total dose administered. In a survey of studies of epidural anesthesia, overt toxicity progressing to convulsions occurred in approximately 0.1% of local anesthetic administrations.
Cardiovascular System Reactions: High doses or unintentional intravascular injection may lead to high plasma levels and related depression of the myocardium, decreased cardiac output, heartblock, hypotension, bradycardia, ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation, and cardiac arrest. (See WARNINGS, PRECAUTIONS, and OVERDOSAGE.)
Allergic: Allergic-type reactions are rare and may occur as a result of sensitivity to the local anesthetic or to other formulation ingredients, such as the antimicrobial preservative methylparaben contained in multiple-dose vials or sulfites in epinephrine-containing solutions. These reactions are characterized by signs such as urticaria, pruritus, erythema, angioneurotic edema (including laryngeal edema), tachycardia, sneezing, nausea, vomiting, dizziness, syncope, excessive sweating, elevated temperature, and possibly, anaphylactoid-like symptomatology (including severe hypotension). Cross sensitivity among members of the amide-type local anesthetic group has been reported. The usefulness of screening for sensitivity has not been definitely established.
Neurologic: The incidences of adverse neurologic reactions associated with the use of local anesthetics may be related to the total dose of local anesthetic administered and are also dependent upon the particular drug used, the route of administration, and the physical status of the patient. Many of these effects may be related to local anesthetic techniques, with or without a contribution from the drug.
In the practice of caudal or lumbar epidural block, occasional unintentional penetration of the subarachnoid space by the catheter or needle may occur. Subsequent adverse effects may depend partially on the amount of drug administered intrathecally and the physiological and physical effects of a dural puncture. A high spinal is characterized by paralysis of the legs, loss of consciousness, respiratory paralysis, and bradycardia.
Neurologic effects following epidural or caudal anesthesia may include spinal block of varying magnitude (including high or total spinal block); hypotension secondary to spinal block; urinary retention; fecal and urinary incontinence; loss of perineal sensation and sexual function; persistent anesthesia, paresthesia, weakness, paralysis of the lower extremities and loss of sphincter control all of which may have slow, incomplete, or no recovery; headache; backache; septic meningitis; meningismus; slowing of labor; increased incidence of forceps delivery; and cranial nerve palsies due to traction on nerves from loss of cerebrospinal fluid.
Neurologic effects following other procedures or routes of administration may include persistent anesthesia, paresthesia, weakness, paralysis, all of which may have slow, incomplete, or no recovery.
-
OVERDOSAGE
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local anesthetic solution. (See ADVERSE REACTIONS, WARNINGS, and PRECAUTIONS.)
Management of Local Anesthetic Emergencies: The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient’s state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be administered.
The first step in the management of systemic toxic reactions, as well as underventilation or apnea due to unintentional subarachnoid injection of drug solution, consists of immediate attention to the establishment and maintenance of a patent airway and effective assisted or controlled ventilation with 100% oxygen with a delivery system capable of permitting immediate positive airway pressure by mask. This may prevent convulsions if they have not already occurred.
If necessary, use drugs to control the convulsions. A 50 mg to 100 mg bolus IV injection of succinylcholine will paralyze the patient without depressing the central nervous or cardiovascular systems and facilitate ventilation. A bolus IV dose of 5 mg to 10 mg of diazepam or 50 mg to 100 mg of thiopental will permit ventilation and counteract central nervous system stimulation, but these drugs also depress central nervous system, respiratory, and cardiac function, add to postictal depression and may result in apnea. Intravenous barbiturates, anticonvulsant agents, or muscle relaxants should only be administered by those familiar with their use. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated. Supportive treatment of circulatory depression may require administration of intravenous fluids, and when appropriate, a vasopressor dictated by the clinical situation (such as ephedrine or epinephrine to enhance myocardial contractile force).
Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated after initial administration of oxygen by mask if difficulty is encountered in the maintenance of a patent airway, or if prolonged ventilatory support (assisted or controlled) is indicated.
Recent clinical data from patients experiencing local anesthetic-induced convulsions demonstrated rapid development of hypoxia, hypercarbia, and acidosis with bupivacaine within a minute of the onset of convulsions. These observations suggest that oxygen consumption and carbon dioxide production are greatly increased during local anesthetic convulsions and emphasize the importance of immediate and effective ventilation with oxygen which may avoid cardiac arrest.
If not treated immediately, convulsions with simultaneous hypoxia, hypercarbia, and acidosis plus myocardial depression from the direct effects of the local anesthetic may result in cardiac arrhythmias, bradycardia, asystole, ventricular fibrillation, or cardiac arrest. Respiratory abnormalities, including apnea, may occur. Underventilation or apnea due to unintentional subarachnoid injection of local anesthetic solution may produce these same signs and also lead to cardiac arrest if ventilatory support is not instituted. If cardiac arrest should occur, successful outcome may require prolonged resuscitative efforts.
The supine position is dangerous in pregnant women at term because of aortocaval compression by the gravid uterus. Therefore during treatment of systemic toxicity, maternal hypotension or fetal bradycardia following regional block, the parturient should be maintained in the left lateral decubitus position if possible, or manual displacement of the uterus off the great vessels be accomplished.
The mean seizure dosage of bupivacaine in rhesus monkeys was found to be 4.4 mg/kg with mean arterial plasma concentration of 4.5 mcg/mL. The intravenous and subcutaneous LD50 in mice is 6 mg/kg to 8 mg/kg and 38 mg/kg to 54 mg/kg respectively.
-
DOSAGE AND ADMINISTRATION
The dose of any local anesthetic administered varies with the anesthetic procedure, the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, the depth of anesthesia and degree of muscle relaxation required, the duration of anesthesia desired, individual tolerance, and the physical condition of the patient. The smallest dose and concentration required to produce the desired result should be administered. Dosages of MARCAINE should be reduced for elderly and/or debilitated patients and patients with cardiac and/or liver disease. The rapid injection of a large volume of local anesthetic solution should be avoided and fractional (incremental) doses should be used when feasible.
For specific techniques and procedures, refer to standard textbooks.
There have been adverse event reports of chondrolysis in patients receiving intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures. MARCAINE is not approved for this use (see WARNINGS and DOSAGE AND ADMINISTRATION).
In recommended doses, MARCAINE produces complete sensory block, but the effect on motor function differs among the three concentrations.
0.25%—when used for caudal, epidural, or peripheral nerve block, produces incomplete motor block. Should be used for operations in which muscle relaxation is not important, or when another means of providing muscle relaxation is used concurrently. Onset of action may be slower than with the 0.5% or 0.75% solutions.
0.5%— provides motor blockade for caudal, epidural, or nerve block, but muscle relaxation may be inadequate for operations in which complete muscle relaxation is essential.
0.75%—produces complete motor block. Most useful for epidural block in abdominal operations requiring complete muscle relaxation, and for retrobulbar anesthesia. Not for obstetrical anesthesia.
The duration of anesthesia with MARCAINE is such that for most indications, a single dose is sufficient.
Maximum dosage limit must be individualized in each case after evaluating the size and physical status of the patient, as well as the usual rate of systemic absorption from a particular injection site. Most experience to date is with single doses of MARCAINE up to 225 mg with epinephrine 1:200,000 and 175 mg without epinephrine; more or less drug may be used depending on individualization of each case.
These doses may be repeated up to once every three hours. In clinical studies to date, total daily doses have been up to 400 mg. Until further experience is gained, this dose should not be exceeded in 24 hours. The duration of anesthetic effect may be prolonged by the addition of epinephrine.
The dosages in Table 1 have generally proved satisfactory and are recommended as a guide for use in the average adult. These dosages should be reduced for elderly or debilitated patients. Until further experience is gained, MARCAINE is not recommended for pediatric patients younger than 12 years. MARCAINE is contraindicated for obstetrical paracervical blocks, and is not recommended for intravenous regional anesthesia (Bier Block).
Use in Epidural Anesthesia: During epidural administration of MARCAINE, 0.5% and 0.75% solutions should be administered in incremental doses of 3 mL to 5 mL with sufficient time between doses to detect toxic manifestations of unintentional intravascular or intrathecal injection. In obstetrics, only the 0.5% and 0.25% concentrations should be used; incremental doses of 3 mL to 5 mL of the 0.5% solution not exceeding 50 mg to 100 mg at any dosing interval are recommended. Repeat doses should be preceded by a test dose containing epinephrine if not contraindicated. Use only the single-dose ampuls and single-dose vials for caudal or epidural anesthesia; the multiple-dose vials contain a preservative and therefore should not be used for these procedures.
Test Dose for Caudal and Lumbar Epidural Blocks: The Test Dose of MARCAINE (0.5% bupivacaine with 1:200,000 epinephrine in a 3 mL ampul) is recommended for use as a test dose when clinical conditions permit prior to caudal and lumbar epidural blocks. This may serve as a warning of unintended intravascular or subarachnoid injection. (See PRECAUTIONS.) The pulse rate and other signs should be monitored carefully immediately following each test dose administration to detect possible intravascular injection, and adequate time for onset of spinal block should be allotted to detect possible intrathecal injection. An intravascular or subarachnoid injection is still possible even if results of the test dose are negative. The test dose itself may produce a systemic toxic reaction, high spinal or cardiovascular effects from the epinephrine. (See WARNINGS and OVERDOSAGE.)
Use in Dentistry: The 0.5% concentration with epinephrine is recommended for infiltration and block injection in the maxillary and mandibular area when a longer duration of local anesthetic action is desired, such as for oral surgical procedures generally associated with significant postoperative pain. The average dose of 1.8 mL (9 mg) per injection site will usually suffice; an occasional second dose of 1.8 mL (9 mg) may be used if necessary to produce adequate anesthesia after making allowance for 2 to 10 minutes onset time. (See CLINICAL PHARMACOLOGY.) The lowest effective dose should be employed and time should be allowed between injections; it is recommended that the total dose for all injection sites, spread out over a single dental sitting, should not ordinarily exceed 90 mg for a healthy adult patient (ten 1.8 mL injections of 0.5% MARCAINE with epinephrine). Injections should be made slowly and with frequent aspirations. Until further experience is gained, MARCAINE in dentistry is not recommended for pediatric patients younger than 12 years.
Unused portions of solution not containing preservatives, i.e., those supplied in single-dose ampuls and single-dose vials, should be discarded following initial use.
This product should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Solutions which are discolored or which contain particulate matter should not be administered.
Table 1. Recommended Concentrations and Doses of MARCAINE Type of
BlockConc.
Each Dose
Motor
Block1(mL)
(mg)
Local
infiltration
0.25%4
up to
max.up to
max.—
Epidural
0.75%2,4
10-20
75-150
complete
0.5%4
10-20
50-100
moderate
to complete0.25%4
10-20
25-50
partial
to moderateCaudal
0.5%4
15-30
75-150
moderate
to complete0.25%4
15-30
37.5-75
moderate
Peripheral
nerves
0.5%4
5 to
max.25 to
max.moderate
to complete0.25%4
5 to
max.12.5 to
max.moderate
to completeRetrobulbar3
0.75%4
2-4
15-30
complete
Sympathetic
0.25%
20-50
50-125
—
Dental3
0.5%
w/epi1.8-3.6
per site9-18
per site—
Epidural3
Test Dose0.5%
w/epi2-3
10-15
(10-15 micrograms epinephrine)—
1With continuous (intermittent) techniques, repeat doses increase the degree of motor block. The first repeat dose of 0.5% may produce complete motor block. Intercostal nerve block with 0.25% may also produce complete motor block for intra-abdominal surgery.
2For single-dose use, not for intermittent epidural technique. Not for obstetrical anesthesia.
3See PRECAUTIONS.
4Solutions with or without epinephrine.
-
HOW SUPPLIED
These solutions are not for spinal anesthesia.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
MARCAINE ―Solutions of MARCAINE that do not contain epinephrine may be autoclaved. Autoclave at 15-pound pressure, 121°C (250°F) for 15 minutes.
NDC No.
Container
Fill
Quantity
0.25%—Contains 2.5 mg bupivacaine hydrochloride per mL.
0409-1559-10
Single-dose vials
10 mL
box of 10
0409-1559-30
Single-dose vials
30 mL
box of 10
0409-1587-50
Multiple-dose vials
50 mL
box of 1
0.5%—Contains 5 mg bupivacaine hydrochloride per mL.
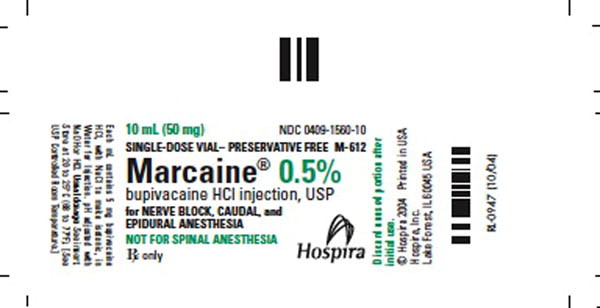
0409-1560-10
Single-dose vials
10 mL
box of 10
0409-1560-29
Single-dose vials
30 mL
box of 10
0409-1610-50
Multiple-dose vials
50 mL
box of 1
0.75%—Contains 7.5 mg bupivacaine hydrochloride per mL.
0409-1582-10
Single-dose vials
10 mL
box of 10
0409-1582-29
Single-dose vials
30 mL
box of 10
MARCAINE with epinephrine 1:200,000 (as bitartrate)―Solutions of MARCAINE that contain epinephrine should not be autoclaved and should be protected from light. Do not use the solution if its color is pinkish or darker than slightly yellow or if it contains a precipitate.
NDC No.
Container
Fill
Quantity
0.25% with epinephrine 1:200,000—Contains 2.5 mg bupivacaine hydrochloride per mL.
0409-1746-10
Single-dose vials
10 mL
box of 10
0409-1746-30
Single-dose vials
30 mL
box of 10
0409-1752-50
Multiple-dose vials
50 mL
box of 1
0.5% with epinephrine 1:200,000—Contains 5 mg bupivacaine hydrochloride per mL.0409-1749-03
Single-dose ampuls
3 mL
box of 10
0409-1749-10
Single-dose vials
10 mL
box of 10
0409-1749-29
Single-dose vials
30 mL
box of 10
0409-1755-50
Multiple-dose vials
50 mL
box of 1
Revised: 10/2011
Printed in USA EN-2916
Hospira, Inc., Lake Forest, IL 60045 USA
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Methylprednisolone acetate injectable suspension, USP is an anti-inflammatory glucocorticoid for intramuscular, intra-articular, soft tissue or intralesional injection. It is available in two strengths: 40 mg/mL; 80 mg/mL
Each mL of these preparations contains:
Methylprednisolone acetate…………………………………..
40 mg
80 mg
Polyethylene glycol 3350……………………………………..
29.1 mg
28.2 mg
Polysorbate 80…………………………………….………...
1.94 mg
1.88 mg
Monobasic sodium phosphate………………………………..
6.8 mg
6.59 mg
Dibasic sodium phosphate USP………………………………
1.42 mg
1.37 mg
Benzyl alcohol added as a preservative……………………….
9.16 mg
8.88 mg
Sodium Chloride was added to adjust tonicity.
When necessary, pH was adjusted with sodium hydroxide and/or hydrochloric acid.
The pH of the finished product remains within the USP specified range; e.g., 3.0 to 7.0.
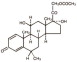
The chemical name for methylprednisolone acetate is pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-11,17-dihydroxy-6-methyl-,(6α,11β)-and the molecular weight is 416.51. The structural formula is represented below:
Methylprednisolone acetate injectable suspension, USP contains methylprednisolone acetate which is the 6-methyl derivative of prednisolone. Methylprednisolone acetate is a white or practically white, odorless, crystalline powder which melts at about 215° with some decomposition. It is soluble in dioxane, sparingly soluble in acetone, in alcohol, in chloroform, and in methanol, and slightly soluble in ether. It is practically insoluble in water.
-
CLINICAL PHARMACOLOGY
Glucocorticoids, naturally occurring and synthetic are adrenocortical steroids.
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt retaining properties, are used in replacement therapy in adrenocortical deficiency states. Their synthetic analogs are used primarily for their anti-inflammatory effects in disorders of many organ systems.
Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body's immune response to diverse stimuli.
-
INDICATIONS AND USAGE
A. For Intramuscular Administration
When oral therapy is not feasible and the strength, dosage form, and route of administration of the drug reasonably lend the preparation to the treatment of the condition, the intramuscular use of methylprednisolone acetate injectable suspension, USP is indicated as follows:
Allergic States: Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in asthma, atopic dermatitis, contact dermatitis, drug hypersensitivity reactions, seasonal or perennial allergic rhinitis, serum sickness, transfusion reactions.
Dermatologic Diseases: Bullous dermatitis herpetiformis, exfoliative erythroderma, mycosis fungoides, pemphigus, severe erythema multiforme (Stevens-Johnson syndrome).
Endocrine Disorders: Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the drug of choice; synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy, mineralocorticoid supplementation is of particular importance) congenital adrenal hyperplasia, hypercalcemia associated with cancer, nonsuppurative thyroiditis.
Gastrointestinal Diseases: To tide the patient over a critical period of the disease in regional enteritis (systemic therapy), ulcerative colitis.
Hematologic Disorders: Acquired (autoimmune) hemolytic anemia, congenital (erythroid) hypoplastic anemia (Diamond blackfan anemia), pure red cell aplasia, select cases of secondary thrombocytopenia.
Miscellaneous: Trichinosis with neurologic or myocardial involvement, tuberculous meningitis with subarachnoid block or impending block when used concurrently with appropriate antituberculous chemotherapy.
Neoplastic Diseases: For palliative management of leukemias and lymphomas.
Nervous System: Acute exacerbations of multiple sclerosis. Cerebral edema associated with primary or metastatic brain tumor or craniotomy.
Ophthalmic Diseases: Sympathetic ophthalmia, temporal arteritis, Uveitis and ocular inflammatory conditions unresponsive to topical corticosteroids.
Renal Diseases: To induce diuresis or remission of proteinuria in idiopathic nephrotic syndrome, or that due to lupus erythematosus.
Respiratory Diseases: Berylliosis, symptomatic sarcoidosis, fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy, idiopathic eosinophilic pneumonias, symptomatic sarcoidosis.
Rheumatic Disorders: As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in acute gouty arthritis, acute rheumatic carditis, ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy). For the treatment of dermatomyositis, polymyositis, and systemic lupus erythematosus.
B. For Intra-articular or Soft Tissue Administration
(See WARNINGS)
Methylprednisolone acetate injectable suspension, USP is indicated as adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in acute gouty arthritis, acute and subacute bursitis, acute nonspecific tenosynovitis, epicondylitis, rheumatoid arthritis, synovitis of osteoarthritis.
C. For Intralesional Administration
Methylprednisolone acetate injectable suspension, USP is indicated for intralesional use in alopecia areata, discoid lupus erythematosus, keloids, localized hypertrophic, infiltrated, inflammatory lesions of granuloma annulare, lichen planus, lichen simplex chronicus (neurodermatitis), and psoriatic plaques, necrobiosis lipoidica diabeticorum.
Methylprednisolone acetate injectable suspension, USP also may be useful in cystic tumors of an aponeurosis or tendon (ganglia).
-
CONTRAINDICATIONS
Methylprednisolone acetate injectable suspension, USP is contraindicated in patients with known hypersensitivity to the product and its constituents.
Intramuscular corticosteroid preparations are contraindicated for idiopathic thrombocytopenic purpura.
Methylprednisolone acetate injectable suspension, USP is contraindicated for intrathecal administration. Reports of severe medical events have been associated with this route of administration.
Methylprednisolone acetate injectable suspension, USP is contraindicated for use in premature infants because the formulation contains benzyl alcohol. (See WARNINGS and PRECAUTIONS:Pediatric Use)
Methylprednisolone acetate injectable suspension, USP is contraindicated in systemic fungal infections, except when administered as an intra-articular injection for localized joint conditions (see WARNINGS, Infections, Fungal Infections)
-
WARNINGS
General
This product contains benzyl alcohol which is potentially toxic when administered locally to neural tissue. Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol. The amount of benzyl alcohol from medications is usually considered negligible compared to that received in flush solutions containing benzyl alcohol. Administration of high dosages of medications containing this preservative must take into account the total amount of benzyl alcohol administered. The amount of benzyl alcohol at which toxicity may occur is not known. If the patient requires more than the recommended dosages or other medications containing this preservative, the practitioner must consider the daily metabolic load of benzyl alcohol from these combined sources (see PRECAUTIONS: Pediatric Use)
Multidose use of methylprednisolone acetate injectable suspension, USP from a single vial requires special care to avoid contamination. Although initially sterile, any multidose use of vials may lead to contamination unless strict aseptic technique is observed. Particular care, such as use of disposable sterile syringes and needles is necessary.
Injection of methylprednisolone acetate injectable suspension, USP may result in dermal and/or subdermal changes forming depressions in the skin at the injection site.
In order to minimize the incidence of dermal and subdermal atrophy, care must be exercised not to exceed recommended doses in injections. Multiple small injections into the area of the lesion should be made whenever possible. The technique of intra-articular and intramuscular injection should include precautions against injection or leakage into the dermis. Injection into the deltoid muscle should be avoided because of a high incidence of subcutaneous atrophy.
It is critical that, during administration of methylprednisolone acetate injectable suspension, USP, appropriate technique be used and care taken to assure proper placement of drug.
Rare instances of anaphylactoid reactions have occurred in patients receiving corticosteroid therapy. (see ADVERSE REACTIONS)
Increased dosage of rapidly acting corticosteroids is indicated in patients on corticosteroid therapy subjected to any unusual stress before, during, or after the stressful situation.
Results from one multicenter, randomized, placebo controlled study with methylprednisolone hemisuccinate, an IV corticosteroid, showed an increase in early (at 2 weeks) and late (at 6 months) mortality in patients with cranial trauma who were determined not to have other clear indications for corticosteroid treatment. High doses of systemic corticosteroids, including methylprednisolone acetate injectable suspension, USP, should not be used for the treatment of traumatic brain injury.
Cardio-renal
Average and large doses of corticosteroids can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Literature reports suggest an apparent association between the use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.
Endocrine
Hypothalamic-pituitary adrenal (HPA) axis suppression. Cushing's syndrome, and hyperglycemia. Monitor patients for these conditions with chronic use.
Corticosteroids can produce reversible HPA axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Drug induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted.
Infections
General
Persons who are on corticosteroids are more susceptible to infections than are healthy individuals. There may be decreased resistance and inability to localize infection when corticosteroids are used. Infections with any pathogen (viral, fungal, protozoan, or helminthic), in any location of the body may be associated with the use of corticosteroids alone or in combination with other immunosuppressive agents.
These infections may be mild, but can be severe and at times fatal. With increasing doses of corticosteroids, the rate of occurrence of infectious complications increases. Corticosteroids may mask some signs of current infection. Do not use intra-articularly, intrabursally or for intratendinous administration for local effect in the presence of acute local infection.
Fungal Infections
Corticosteroids may exacerbate systemic fungal infections and therefore should not be used in the presence of such infections unless they are needed to control drug reactions. There have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure (see CONTRAINDICATIONS and PRECAUTIONS, Drug Interactions, Amphotericin B injections and potassium depleting agents)
Special pathogens
Latent disease may be activated or there may be an exacerbation of intercurrent infections due to pathogens, including those caused by Amoeba, Candida, Cryptococcus, Mycobacterium, Nocardia, Pneumocystis, Taxoplasma.
It is recommended that latent amebiasis or active amebiasis be ruled out before initiating corticosteroid therapy in any patient who has spent time in the tropics or in any patient with unexplained diarrhea.
Similarly, corticosteroids should be used with great care in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Stronglyoides hyperinfection and dissemination with widespread larval migration, often accompanied by severe entercolitis and potentially fatal gram-negative septicemia.
Corticosteroids should not be used in cerebral malaria. There is currently no evidence of benefit from steroids in this condition.
Tuberculosis
The use of corticosteroids in active tuberculosis should be restricted to those cases of fulminating or disseminated tuberculosis in which the corticosteroid is used for the management of the disease in conjunction with an appropriate antituberculous regimen.
If corticosteroids are indicated in patients with latent tuberculosis or tuberculin reactivity, close observation is necessary as reactivation of the disease may occur. During prolonged corticosteroid therapy, these patients should receive chemoprophylaxis.
Vaccinations
Administration of live or live, attenuated vaccines is contraindicated in patients receiving immunosuppressive doses of corticosteroids. Killed or inactivated vaccines may be administered. However, the response to such vaccines can not be predicted. Immunization procedures may be undertaken in patients who are receiving corticosteroids, as replacement therapy, e.g. for Addison's disease.
Viral Infections
Chicken pox and measles can have a more serious or even fatal course in pediatric and adult patients on corticosteroids. In pediatric and adult patients who have not had these diseases, particular care should be taken to avoid exposure. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chicken pox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chicken pox develops, treatment with antiviral agents should be considered.
Ophthalmic
Use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to bacteria, fungi or viruses. The use of systemic corticosteroids is not recommended in the treatment of optic neuritis and may lead to an increase in the risk of new episodes. Corticosteroids should be used cautiously in patients with active ocular herpes simplex because of corneal perforation. Corticosteroids should not be used in active ocular herpes simplex.
-
PRECAUTIONS
General
When multidose vials are used, special care to prevent contamination of the contents is essential. A povidone-iodine solution or similar product is recommended to cleanse the vial top prior to aspiration of contents. (See WARNINGS)
This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not be autoclaved when it is desirable to sterilize the outside of the vial.
The lowest possible dose of corticosteroid should be used to control the condition under treatment. When reduction in dosage is possible, the reduction should be gradual.
Since complications of treatment with glucocorticosteroids are dependent on the size of the dose and duration of treatment, a risk/benefit decision must be made in each individual case as to dose and duration of treatment and as to whether daily or intermittent therapy should be used.
Kaposi's sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement.
Cardio-renal
As sodium retention with resultant edema and potassium loss may occur in patients receiving corticosteroids, these agents should be used with caution in patients with congestive heart failure, hypertension, or renal insufficiency.
Endocrine
Drug-induced secondary adrenocortical insufficiency may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. Since mineral corticosteroid secretion may be impaired, salt and/or a mineral corticosteroid should be administered concurrently.
Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid status of the patient may necessitate adjustment in dosage.
Gastrointestinal
Steroids should be used in caution in active or latent peptic ulcer, diverticulitis, fresh intestinal anastomoses, and non-specific ulcerative colitis, since they may increase the risk of a perforation.
Signs of peritoneal irritation following gastrointestinal perforation in patients receiving corticosteroids may be minimal or absent.
There is an enhanced effect due to increased metabolism of corticosteroids in patients with cirrhosis.
Parenteral Administration
Intra-articular injected corticosteroids may be systemically absorbed.
Appropriate examination of any joint fluid is necessary to exclude a septic process.
A marked increase in pain associated by local swelling, further restriction of joint motion, fever, malaise are suggestive of septic arthritis. If this complication occurs and the diagnosis of sepsis is confirmed, appropriate antimicrobial therapy should be instituted.
Injection of a steroid into an infected site is to be avoided. Local injection of a steroid into a previously infected joint is not usually recommended.
Musculoskeletal
Corticosteroids decrease bone formation and increase bone resorption both through their effect on calcium regulation (e.g., decreasing absorption and increasing excretion) and inhibition of osteoblast function. This, together with a decrease in protein matrix of the bone secondary to an increase in protein catabolism, and reduced sex hormone production, may lead to inhibition of bone growth in pediatric patients and the development of osteoporosis at any age (e.g., postmenopausal women) before initiating corticosteroid therapy.
Neuro-psychiatric
Although controlled clinical trials have shown corticosteroids to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that corticosteroids affect the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of corticosteroids are necessary to demonstrate a significant effect. (See DOSAGE AND ADMINISTRATION)
An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (e.g., myasthenia gravis), or in patients receiving concomitant therapy with neuromuscular blocking drugs (e.g., pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may results in quadriparesis. Elevation of creatine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Ophthalmic
Intraocular pressure may become elevated in some individuals. If steroid therapy is continued for more than 6 weeks, intraocular pressure should be monitored.
Corticosteroids should be used cautiously in patients with ocular herpes simplex for fear of corneal perforation.
Information for the Patient
Patients should be warned not to discontinue the use of corticosteroids abruptly or without medical supervision, to advise any medical attendants that they are taking corticosteroids and to seek medical advice at once should they develop fever or other signs of infection.
Persons who are on corticosteroids should be warned to avoid exposure to chicken pox or measles. Patients should also be advised that if they are exposed, medical advice should be sought without delay.
DRUG INTERACTIONS
Aminoglutethimide
Aminoglutethimide may lead to a loss of corticosteroid-induced adrenal suppression.
Amphotericin B injection and potassium- depleting agents
When corticosteroids are administered concomitantly with potassium-depleting agents (e.g., amphotericin B, diuretics), patients should be observed closely for development of hypokalemia. There have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure.
Antibiotics
Macrolide antibiotics have been reported to cause significant decrease in corticosteroid clearance (see DRUG INTERACTIONS, Hepatic Enzyme Inhibitors)
Anticholinesterases
Concomitant use of anticholinesterase agents and corticosteroids may produce severe weakness in patients with myasthenia gravis. If possible, anticholinesterase agents should be withdrawn at least 24 hours before initiating corticosteroid therapy.
Anticoagulants, oral
Coadministration of corticosteroids and warfarin usually results in inhibition of response to warfarin, although there have been some conflicting reports. Therefore, coagulation indices should be monitored frequently to maintain the desired anticoagulant effect.
Antidiabetics
Because corticosteroids may increase blood glucose concentrations, dosage adjustments of antidiabetic agents may be required.
Cyclosporine
Increased activity of both cyclosporine and corticosteroids may occur when the two are used concurrently. Convulsions have been reported with concurrent use.
Digitalis glycosides
Patients on digitalis glycosides may be at increased risk of arrhythmias due to hypokalemia.
Estrogens, including oral contraceptives
Estrogens may decrease the hepatic metabolism of certain corticosteroids, thereby increasing their effect.
Hepatic Enzyme Inducers (e.g., barbiturates, phenytoin, carbamazepine, rifampin)
Drugs which induce cytochrome P450 3A4 enzyme activity may enhance the metabolism of corticosteroids and require that the dosage of corticosteroid be increased.
Hepatic Enzyme Inhibitors (e.g., ketoconazole, macrolide antibiotics such as erythromycin and troleandomycin)
Drugs which inhibit cytochrome P450 3A4 have the potential to result in increased plasma concentrations of corticosteroids.
Ketoconazole
Ketoconazole has been reported to significantly decrease the metabolism of certain corticosteroids by up to 60%,, leading to an increased risk of corticosteroid side effects.
Non-steroidal anti-inflammatory agents (NSAIDs)
Concomitant use of aspirin (or other nonsteroidal anti-inflammatory agents) and corticosteroids increases the risk of gastrointestinal side-effects. Aspirin should be used cautiously in conjunction with concurrent use of corticosteroids in hypoprothrombinemia. The clearance of salicylates may be increased with concurrent use of corticosteroids.
Vaccines
Patients on prolonged corticosteroid therapy may exhibit a diminished response to toxoids and live or attenuated vaccines due to inhibition of antibody response. Corticosteroids may also potentiate the replication of some organisms contained in live attenuated vaccines. Routine administration of vaccines or toxoids should be deferred until corticosteroid therapy is discontinued if possible (see WARNINGS, Infections, Vaccinations)
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate studies have been conducted in animals to determine whether corticosteroids have a potential for carcinogenesis or mutagenesis. Steroids may increase or decrease motility and number of spermatozoa in some patients.
Pregnancy
Teratogenic effects
Pregnancy Category C
Corticosteroids have been shown to be teratogenic in many species when given in doses equivalent to human dose. Animal studies in which corticosteroids have been given to pregnant mice, rats, and rabbits, have yielded an increase incidence of cleft palate in the off-spring. There are no adequate and well-controlled studies in pregnant women. Corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born to mothers who have received corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Because of the potential for serious adverse reactions in nursing infants from corticosteroids, a decision should be made whether to continue nursing, or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
This product contains benzyl alcohol as a preservative. Benzyl alcohol, a component of this product, has been associated with serious adverse events and death, particularly in pediatric patients. The "gasping syndrome", (characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages >99 mg/kg/day in neonates and low-birth-weight neonates. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Although normal therapeutic doses of this product deliver amounts of benzyl alcohol that are substantially lower than those reported in association with the "gasping syndrome", the minimum amount of benzyl alcohol at which toxicity may occur is not known. Premature and low-birth-weight infants, as well as patients receiving high dosages, may be more likely to develop toxicity. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources.
The efficacy and safety of corticosteroids in the pediatric population are based on the well-established course of effect of corticosteroids which is similar in pediatric and adult populations. Published studies provide evidence of efficacy and safety in pediatric patients for the treatment of nephritic syndrome (patients >2 years of age), and aggressive lymphomas and leukemias (patients >1 month of age). Other indications for pediatric use of corticosteroids, e.g., severe asthma and wheezing, are based on adequate and well-controlled clinical trials conducted in adults, on the premises that the course of the diseases and their pathophysiology are considered to be substantially similar in both populations.
The adverse effects of corticosteroids in pediatric patients are similar to those in adults (see ADVERSE REACTIONS). Like adults, pediatric patients should be carefully observed with frequent measurements of blood pressure, weight, height, intraocular pressure, and clinical evaluation for the presence of infection, psychosocial disturbances, thromboembolism, peptic ulcers, cataracts, and osteoporosis. Pediatric patients who are treated with corticosteroids by any route, including systemically administered corticosteroids, may experience a decrease in their growth velocity. This negative impact of corticosteroids on growth has been observed at low systemic doses and in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression (e.g., cosyntropin stimulation and basal cortisol plasma levels). Growth velocity may therefore be a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The linear growth of pediatric patients treated with corticosteroids should be monitored, and the potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the ability of treatment alternatives. In order to minimize the potential growth effects of corticosteroids, pediatric patients should be titrated to the lowest effective dose.
Geriatric Use
Clinical studies did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
The following adverse reactions have been reported with methylprednisolone acetate injectable suspension, USP or other corticosteroids:
Allergic reactions: Allergic or hypersensitivity reactions, anaphylactoid reaction, anaphylaxis, angioedema.
Cardiovascular: Bradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, fat embolism, hypertension, hypertrophic cardiomyopthy in premature infants, myocardial rupture following recent mycocardial infarction (see WARNINGS), pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis.
Dermatologic: Acne, allergic dermatitis, cutaneous and subcutaneous atrophy, dry scaly skin, ecchymoses and petechiae, edema, erythema, hyperpigmentation, hypopigmentation, impaired wound healing, increase sweating, rash, sterile abscess, striae, suppressed reactions to skin tests, thin fragile skin, thinning scalp hair, urticaria.
Endocrine: Decreased carbohydrate and glucose tolerance, development of cushingoid state, glycosuria, hirsutism, hypertrichosis, increased requirements for insulin or oral hypoglycemic agents in diabetes, manifestations of latent diabetes mellitus, menstrual irregularities, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery, or illness), suppression of growth in pediatric patients.
Fluid and electrolyte disturbances: Congestive heart failure in susceptible patients, Fluid retention, hypokalemic alkalosis, potassium loss, sodium retention.
Gastrointestinal: Abdominal distention, bowel/bladder dysfunction (after intrathecal administration), elevation in serum liver enzymes levels (usually reversible upon discontinuation), hepatomegaly, increased appetite, nausea, pancreatitis, peptic ulcer with possible subsequent perforation and hemorrhage, perforation of the small and large intestine (particularly in patients with inflammatory bowel disease), ulcerative esophagitis.
Metabolic: Negative nitrogen balance due to protein catabolism.
Musculoskeletal: Aseptic necrosis of femoral and humeral heads, calcinosis (following intra-articular or intra-lesional use), Charcot-like arthropathy, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, postinjection flare (following intra-articular use), steroid myopathy, tendon rupture, vertebral compression fractures.
Neurologic/Psychiatric: Convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema (pseudotumor cerebri) usually following discontinuation of treatment, insomnia, mood swings, neuritis, neuropathy, paresthesia, personality changes, psychic disorders, vertigo.
Ophthalmic: Exophthalmoses, glaucoma, increased intraocular pressure, posterior subcapsular cataracts.
Other: Abnormal fat deposits, decreased resistance to infection, hiccups, increased or decreased motility and number of spermatozoa, injection site infections following non-sterile administration (see WARNINGS), malaise, moon face, weight gain.
The following adverse reactions have been reported with the following routes of administration:
Intrathecal/Epidural: Arachnoiditis, bowel/bladder dysfunction, headache, meningitis, parapareisis/paraplegia, seizures, sensory disturbances.
Intranasal: Allergic reactions, rhinitis, temporary/permanent visual impairment including blindness.
Ophthalmic: Increased intraocular pressure, infection, ocular and periocular inflammation including allergic reactions, residue or slough at injection site, temporary/permanent visual impairment including blindness.
Miscellaneous injection sites: (scalp, tonsillar fauces, sphenopalatine ganglion): Blindness.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
NOTE: CONTAINS BENZYL ALCOHOL (see WARNINGS and PRECAUTIONS: Pediatric Use)
Because of possible physical incompatibilities, methylprednisolone acetate injectable suspension, USP should not be diluted or mixed with other solutions.
The initial dosage of parenterally administered methylprednisolone acetate injectable suspension, USP will vary from 4 to 120 mg depending on the specific disease entity being treated. However, in certain overwhelming, acute, life-threatening situations, administrations in dosages exceeding the usual dosages may be justified and may be in multiples of the oral doses.
It Should Be Emphasized that Dosage Requirements are Variable and Must Be Individualized on the Basis of the Disease Under Treatment and the Response of the Patient. After a favorable response is noted, the proper maintenance dose should be determined by decreasing the initial drug dosage in small increments at appropriate time intervals until the lowest dosage which will maintain an adequate clinical response is reached. Situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient's individual drug responsiveness, and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment. In this latter situation it may be necessary to increase the dosage of the corticosteroid for a period of time consistent with the patient's condition. If after long term therapy the drug is to be stopped, it is recommended that it be withdrawn gradually rather than abruptly.
A. Administration for Local Effect
Therapy with methylprednisolone acetate injectable suspension, USP does not obviate the need for the conventional measures usually employed. Although this method of treatment will ameliorate symptoms, it is in no sense a cure and the hormone has no effect on the cause of the inflammation.
1. Rheumatoid and Osteoarthritis
The dose for intra-articular administration depends upon the size of the joint and varies with the severity of the condition in the individual patient. In chronic cases, injections may be repeated at intervals ranging from one to five or more weeks depending upon the degree of relief obtained from the initial injection. The doses in the following table are given as a general guide:
Size of Joint Examples Range of Dosage Large
Knees
Ankles
Shoulders20 to 80 mg
Medium
Elbows
Wrists10 to 40 mg
Small
Metacarpophalangeal Interphalangeal
Sternoclavicular Acromioclavicular4 to 10 mg
Procedure
It is recommended that the anatomy of the joint involved be reviewed before attempting intra-articular injection. In order to obtain the full anti-inflammatory effect it is important that the injection be made into the synovial space. Employing the same sterile technique as for a lumbar puncture, a sterile 20 to 24 gauge needle (on a dry syringe) is quickly inserted into the synovial cavity. Procaine infiltration is elective. The aspiration of only a few drops of joint fluid proves the joint space has been entered by the needle. The injection site for each joint is determined by that location where the synovial cavity is most superficial and most free of large vessels and nerves. With the needle in place, the aspirating syringe is removed and replaced by a second syringe containing the desired amount of methylprednisolone acetate injectable suspension, USP. The plunger is then pulled outward slightly to aspirate synovial fluid and to make sure the needle is still in the synovial space. After injection, the joint is moved gently a few times to aid mixing of the synovial fluid and the suspension. The site is covered with a small sterile dressing.
Suitable sites for intra-articular injection are the knee, ankle, wrist, elbow, shoulder, phalangeal, and hip joints. Since difficulty is not infrequently encountered in entering the hip joint, precautions should be taken to avoid any large blood vessels in the area. Joints not suitable for injection are those that are anatomically inaccessible such as the spinal joints and those like the sacroiliac joints that are devoid of synovial space. Treatment failures are most frequently the result of failure to enter the joint space. Little or no benefit follows injection into surrounding tissue. If failures occur when injections into the synovial spaces are certain, as determined by aspiration of fluid, repeated injections are usually futile.
If a local anesthetic is used prior to injection of methylprednisolone acetate injectable suspension, USP, the anesthetic package insert should be read carefully and all the precautions observed.
2. Bursitis
The area around the injection site is prepared in a sterile way and a wheal at the site made with 1 percent procaine hydrochloride solution. A 20 to 24 gauge needle attached to a dry syringe is inserted into the bursa and the fluid aspirated. The needle is left in place and the aspirating syringe changed for a small syringe containing the desired dose. After injection, the needle is withdrawn and a small dressing applied.
3. Miscellaneous: Ganglion, Tendinitis, Epicondylitis
In the treatment of conditions such as tendinitis or tenosynovitis, care should be taken, following application of a suitable antiseptic to the overlying skin, to inject the suspension into the tendon sheath rather than into the substance of the tendon. The tendon may be readily palpated when placed on a stretch. When treating conditions such as epicondylitis, the area of greatest tenderness should be outlined carefully and the suspension infiltrated into the area. For ganglia of the tendon sheaths, the suspension is injected directly into the cyst. In many cases, a single injection causes a marked decrease in the size of the cystic tumor and may effect disappearance. The usual sterile precautions should be observed, of course, with each injection.
The dose in the treatment of the various conditions of the tendinous or bursal structures listed above varies with the condition being treated and ranges from 4 to 30 mg. In recurrent or chronic conditions, repeated injections may be necessary.
4. Injections for Local Effect in Dermatologic Conditions
Following cleansing with an appropriate antiseptic such as 70% alcohol, 20 to 60 mg of the suspension is injected into the lesion. It may be necessary to distribute doses ranging from 20 to 40 mg by repeated local injections in the case of large lesions. Care should be taken to avoid injection of sufficient material to cause blanching since this may be followed by a small slough. One to four injections are usually employed, the intervals between injections varying with the type of lesion being treated and the duration of improvement produced by the initial injection.
When multidose vials are used, special care to prevent contamination of the contents is essential. (See WARNINGS)
B. Administration for Systemic Effect
The intramuscular dosage will vary with the condition being treated. When employed as a temporary substitute for oral therapy, a single injection during each 24-hour period of a dose of the suspension equal to the total daily oral dose of methylprednisolone tablets, USP is usually sufficient. When a prolonged effect is desired, the weekly dose may be calculated by multiplying the daily oral dose by 7 and given as a single intramuscular injection.
In pediatric patients, the initial dose of methylprednisolone may vary depending upon the specific disease entity being treated. The range of initial doses is 0.11 to 1.6 mg/kg/day. Dosage must be individualized according to the severity of the disease and response of the patient.
In patients with the adrenogenital syndrome, a single intramuscular injection of 40 mg every two weeks may be adequate. For maintenance of patients with rheumatoid arthritis, the weekly intramuscular dose will vary from 40 to 120 mg. The usual dosage for patients with dermatologic lesions benefited by systemic corticoid therapy is 40 to 120 mg of methylprednisolone acetate administered intramuscularly at weekly intervals for one to four weeks. In acute severe dermatitis due to poison ivy, relief may result within 8 to 12 hours following intramuscular administration of a single dose of 80 to 120 mg. In chronic contact dermatitis repeated injections at 5 to 10 day intervals may be necessary. In seborrheic dermatitis, a weekly dose of 80 mg may be adequate to control the condition.
Following intramuscular administration of 80 to 120 mg to asthmatic patients, relief may result within 6 to 48 hours and persist for several days to two weeks. Similarly in patients with allergic rhinitis (hay fever) an intramuscular dose of 80 to 120 mg may be followed by relief of coryzal symptoms within six hours persisting for several days to three weeks.
If signs of stress are associated with the condition being treated, the dosage of the suspension should be increased. If a rapid hormonal effect of maximum intensity is required, the intravenous administration of highly soluble methylprednisolone sodium succinate is indicated.
In treatment of acute exacerbations of multiple sclerosis daily doses of 160 mg of methylprednisolone for a week followed by 64 mg every other day for 1 month have been shown to be effective.
For the purpose of comparison, the following is the equivalent milligram dose of the various glucocorticoids:
Cortisone, 25
Triamcinolone, 4
Hydrocortisone, 20
Paramethasone, 2
Prednisolone, 5
Netamethasone, 0.75
Prednisone, 5
Dexamethasone, 0.75
Methylprednisolone, 4
Those dose relationships apply only to oral or intravenous administration of these compounds. When these substances or their derivatives are injected intramuscularly or into joint spaces, their relative properties may be greatly altered.
-
HOW SUPPLIED
Methylprednisolone acetate injectable suspension, USP is available in the following strengths and package sizes:
40 mg per mL
NDC 0703-0043-01
5 mL multidose vials, packaged individually
NDC 0703-0045-01
10 mL multidose vials, packaged individually
80 mg per mL
NDC 0703-0063-01
5 mL multidose vials, packaged individually
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- •
- in the eyes
- •
- on individuals who are allergic or sensitive to iodine
- •
- over large areas of the body
- •
- as a first aid antiseptic longer than one week unless directed by a doctor
Discontinue use if irritation and redness develop. If condition persist for more than 72 hours consult a doctor.
Consult a doctor in case of
- •
- Deep or puncture wounds
- •
- animal bites
- •
- serious burns.
-
Directions
For the preparation of the skin prior to surgery
- •
- clean the treatment area
- •
- Remove swab by stick
- •
- apply to the operative site prior to surgery
For use as a first aid antiseptic
- •
- clean the treatment area
- •
- apply a small amount of this product on the treatment area 1-3 times daily
- •
- may be covered with a sterile bandage
- •
- if bandaged, let dry first
- Other information
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – SWAB LABEL
PDI®
REORDER NO. C124
NDC 10819-3883-3
Povidone-Iodine Prep Pad
Antiseptic/Germicide Large
For Professional and Hospital Use
Professional Disposables International, Inc.
Orangeburg, NY 10962-1376 • USA • 1-800-999-6423
www.pdipdi.com Made in USA
1 Prep Pad [1.7 x 3.5 in (4.4 x 8.8 cm)]
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - KIT CARTON
NDC 76420-0520-01 RX-Only
Physicians EZ Use
M-pred
Injection KitKit Contains:
1 Marcaine® 0.5% Single Dose Vial (10mL)*
1 Methylprednisone Acetate Injection Suspension,
USP 40mg/mL Multiple Dose Vial (5mL)*
1 Povidone-Iodine Prep Pad1 Pair Sterile Latex Gloves - Size 7*
1 CSR Wrap1 Dose
Relabeled By:
Proficient Rx LP Thousand Oaks, CA 91320
-
INGREDIENTS AND APPEARANCE
PHYSICIANS EZ USE M-PRED
methylprednisolone acetate, bupivacaine hydrochloride, povidone-iodine kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63187-352(NDC:76420-520) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63187-352-01 1 in 1 CARTON; Type 0: Not a Combination Product 09/01/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, MULTI-DOSE 5 mL Part 2 1 VIAL, SINGLE-DOSE 10 mL Part 3 1 PACKET 2.2 g Part 1 of 3 METHYLPREDNISOLONE ACETATE
methylprednisolone acetate injection, suspensionProduct Information Item Code (Source) NDC:0703-0043 Route of Administration INTRAMUSCULAR, INTRASYNOVIAL, SOFT TISSUE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYLPREDNISOLONE ACETATE (UNII: 43502P7F0P) (METHYLPREDNISOLONE - UNII:X4W7ZR7023) METHYLPREDNISOLONE ACETATE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) 29.1 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1.94 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) 6.8 mg in 1 mL SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) 1.42 mg in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 9.16 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0703-0043-01 5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040620 10/31/2006 Part 2 of 3 MARCAINE
bupivacaine hydrochloride injection, solutionProduct Information Item Code (Source) NDC:0409-1560 Route of Administration EPIDURAL, INFILTRATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE HYDROCHLORIDE (UNII: 7TQO7W3VT8) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-1560-10 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA016964 05/04/2010 Part 3 of 3 PVP IODINE PREP PAD MEDIUM AND LARGE
povidone iodine swabProduct Information Item Code (Source) NDC:10819-3883 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10819-3883-3 2.2 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 01/01/1978 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA016964 05/30/2013 Labeler - Proficient Rx LP (079196022) Establishment Name Address ID/FEI Business Operations Proficient Rx LP 079196022 REPACK(63187-352) , RELABEL(63187-352)