Label: EQUATE IBUPROFEN- ibuprofen capsule, liquid filled
-
Contains inactivated NDC Code(s)
NDC Code(s): 49035-133-12, 49035-133-14, 49035-133-18, 49035-133-26 - Packager: Walmart stores Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated July 1, 2014
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
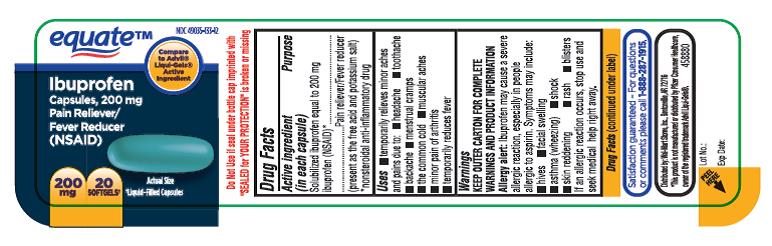
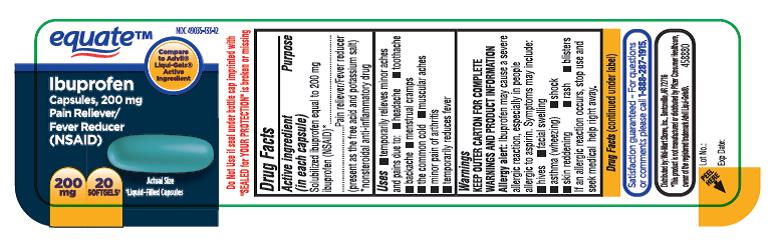
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
-
WARNINGS
Allergy alert:
Ibuprofenmay cause a severe allergic reaction, especially in people allergic to aspirin.Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If anallergic reaction occurs, stop use and seek medical help right away.
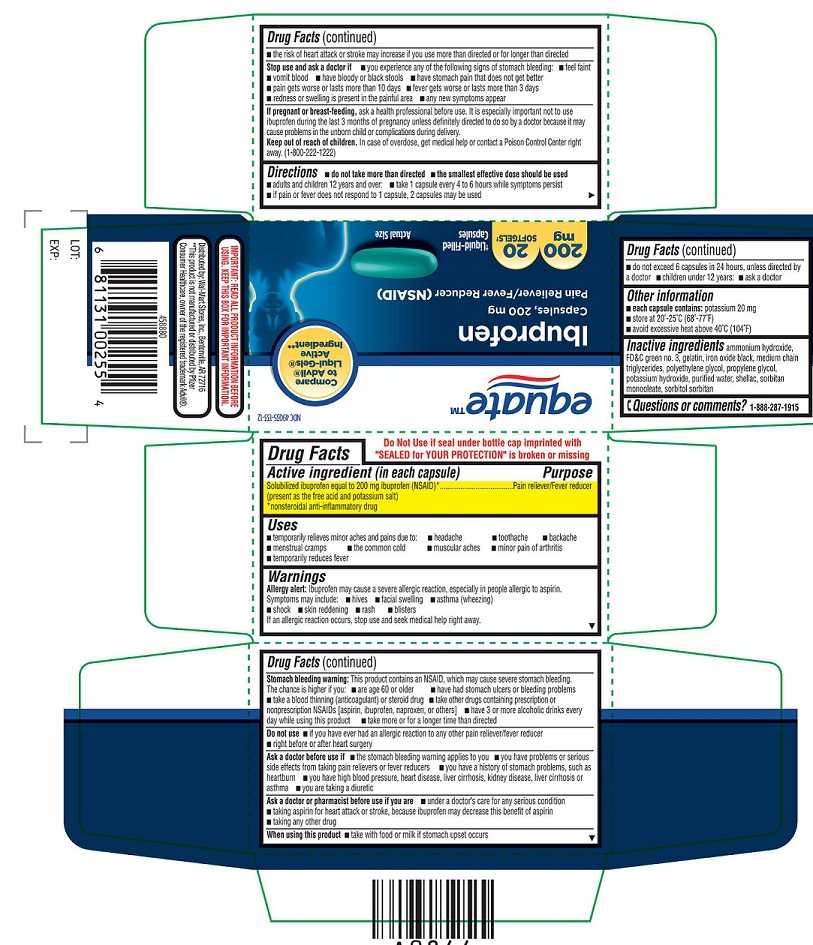
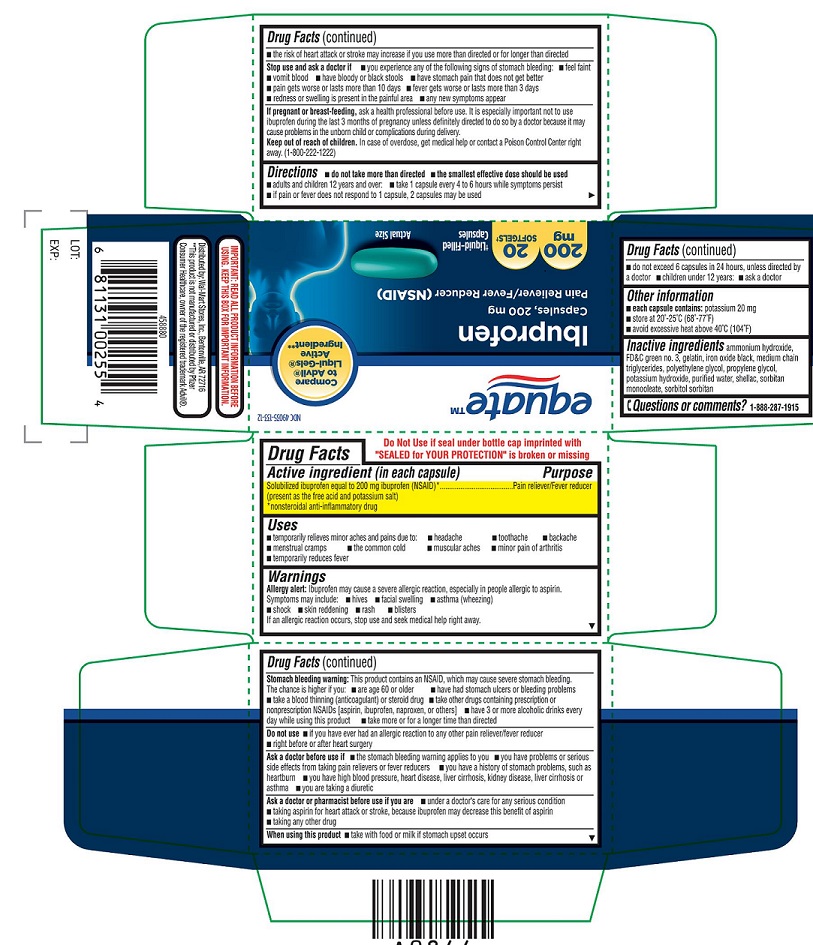
Thisproduct contains an NSAID, which may cause severe stomach bleeding. The chanceis higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- DO NOT USE
-
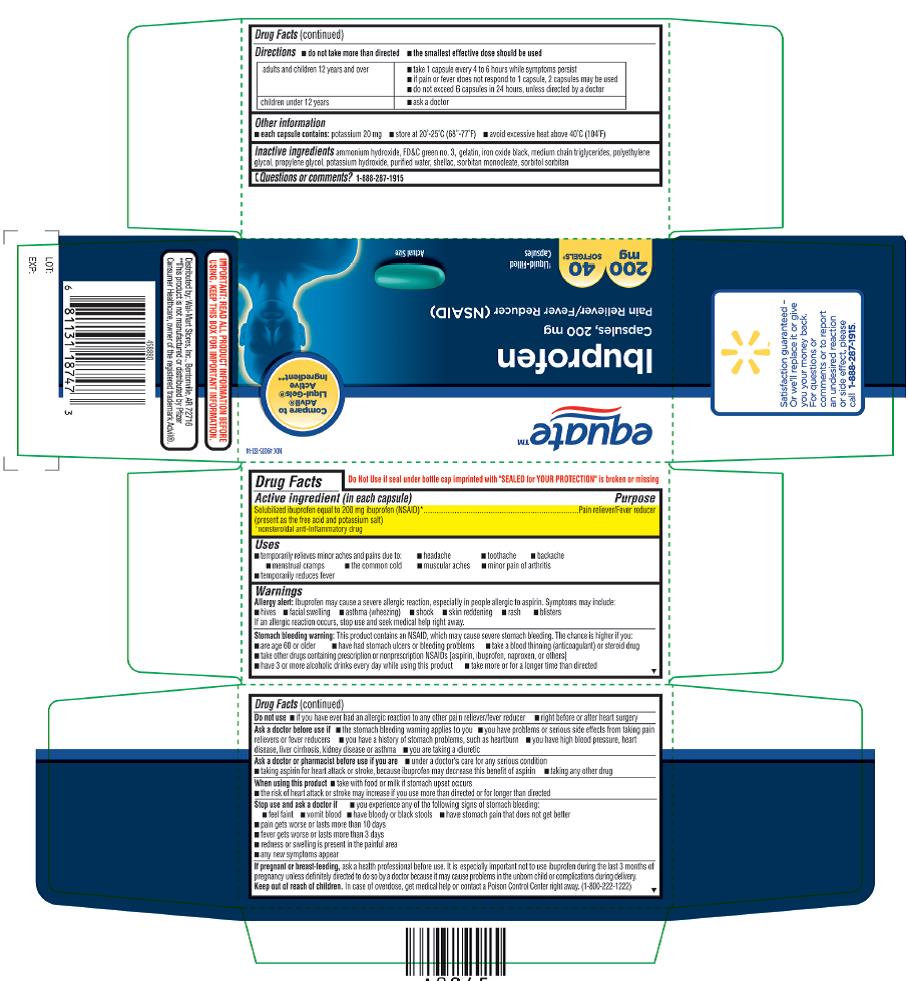
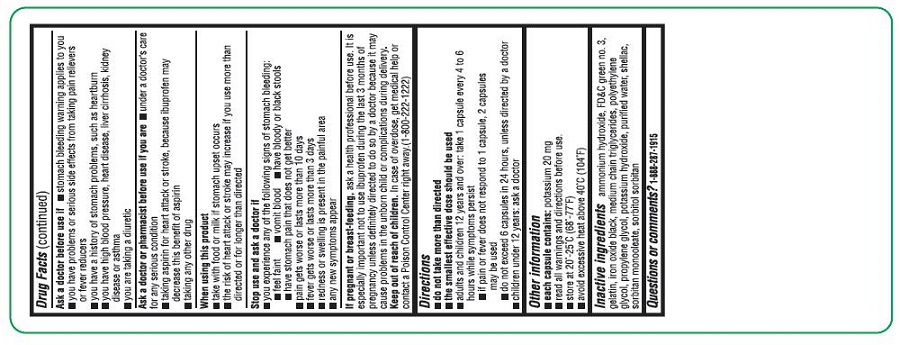
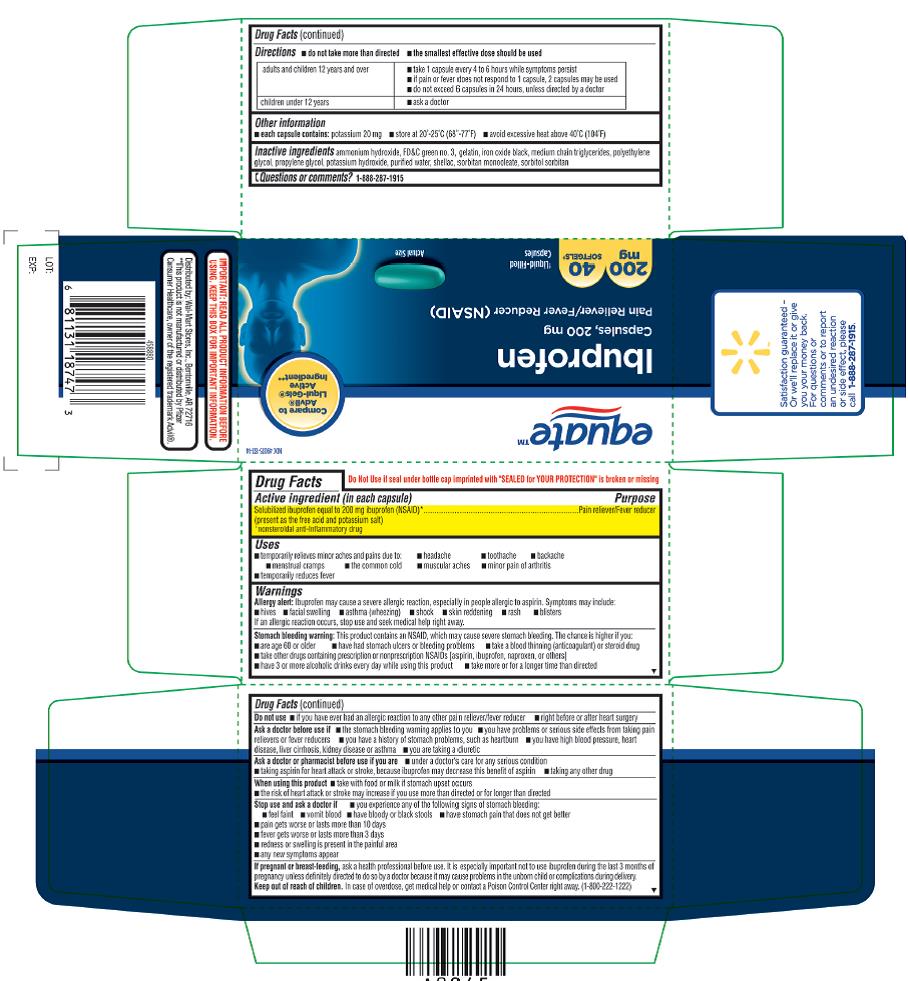
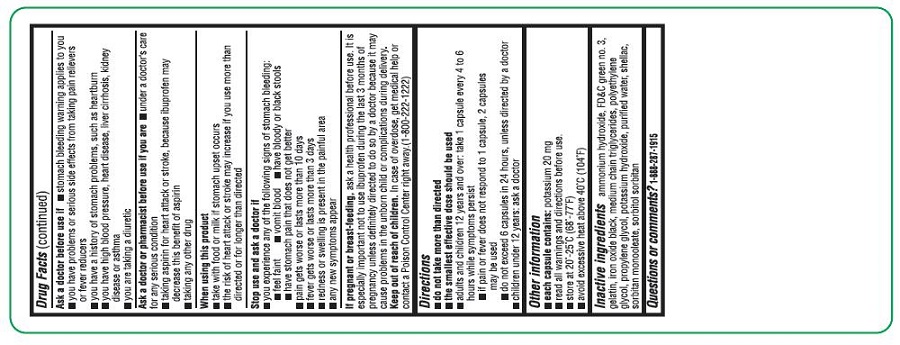
ASK A DOCTOR BEFORE USE IF
- the stomach bleeding warning applies to you
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, or asthma
- you are taking a diuretic
- ASK A DOCTOR OR PHARMACIST BEFORE USE IF
- WHEN USING THIS PRODUCT
-
STOP USE AND ASK DOCTOR IF
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
- OTHER REQUIRED WARNINGS
- PREGNANCY/BREASTFEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- do not take more than directed
- the smallest effective dose should be used
- adults and children 12 years and over: take 1 capsule every 4 to 6 hours while symptoms persist
- if pain or fever does not respond to 1 capsule, 2 capsules may be used
- do not exceed 6 capsules in 24 hours, unless directed by a doctor
- children under 12 years: ask a doctor
- INACTIVE INGREDIENT(S)
-
PRINCIPAL DISPLAY PANEL
NDC 49035-133-12
equateTM
Ibuprofen
Capsules, 200mg
Pain Reliever/ Fever Reducer
(NSAID)
200mg, 20 SOFTGELS
Liquid-Filled Capsules

NDC 49035-133-14
equateTM
Ibuprofen
Capsules, 200mg
Pain Reliever/ Fever
Reducer (NSAID)
Liquid-Filled Capsules
200mg, 40 SOFTGELS


NDC 49035-133-18
equateTM
Ibuprofen
Capsules, 200mg
Pain Reliever/ Fever
Reducer (NSAID)
Liquid-Filled Capsules
200mg, 80 SOFTGELS


NDC 49035-133-26
equateTM
Ibuprofen
Capsules, 200mg
Pain Reliever/ Fever
Reducer (NSAID)
Liquid-Filled Capsules
200mg, 160 SOFTGELS

-
INGREDIENTS AND APPEARANCE
EQUATE IBUPROFEN
ibuprofen capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-133 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) GELATIN (UNII: 2G86QN327L) FERROSOFERRIC OXIDE (UNII: XM0M87F357) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) WATER (UNII: 059QF0KO0R) SHELLAC (UNII: 46N107B71O) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) SORBITOL (UNII: 506T60A25R) SORBITAN (UNII: 6O92ICV9RU) Product Characteristics Color GREEN Score no score Shape CAPSULE Size 19mm Flavor Imprint Code 133 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-133-12 20 in 1 BOTTLE 2 NDC:49035-133-14 40 in 1 BOTTLE 3 NDC:49035-133-18 80 in 1 BOTTLE 4 NDC:49035-133-26 160 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079205 07/02/2014 Labeler - Walmart stores Inc (051957769) Establishment Name Address ID/FEI Business Operations Marksans Pharma Limited 925822975 MANUFACTURE(49035-133)