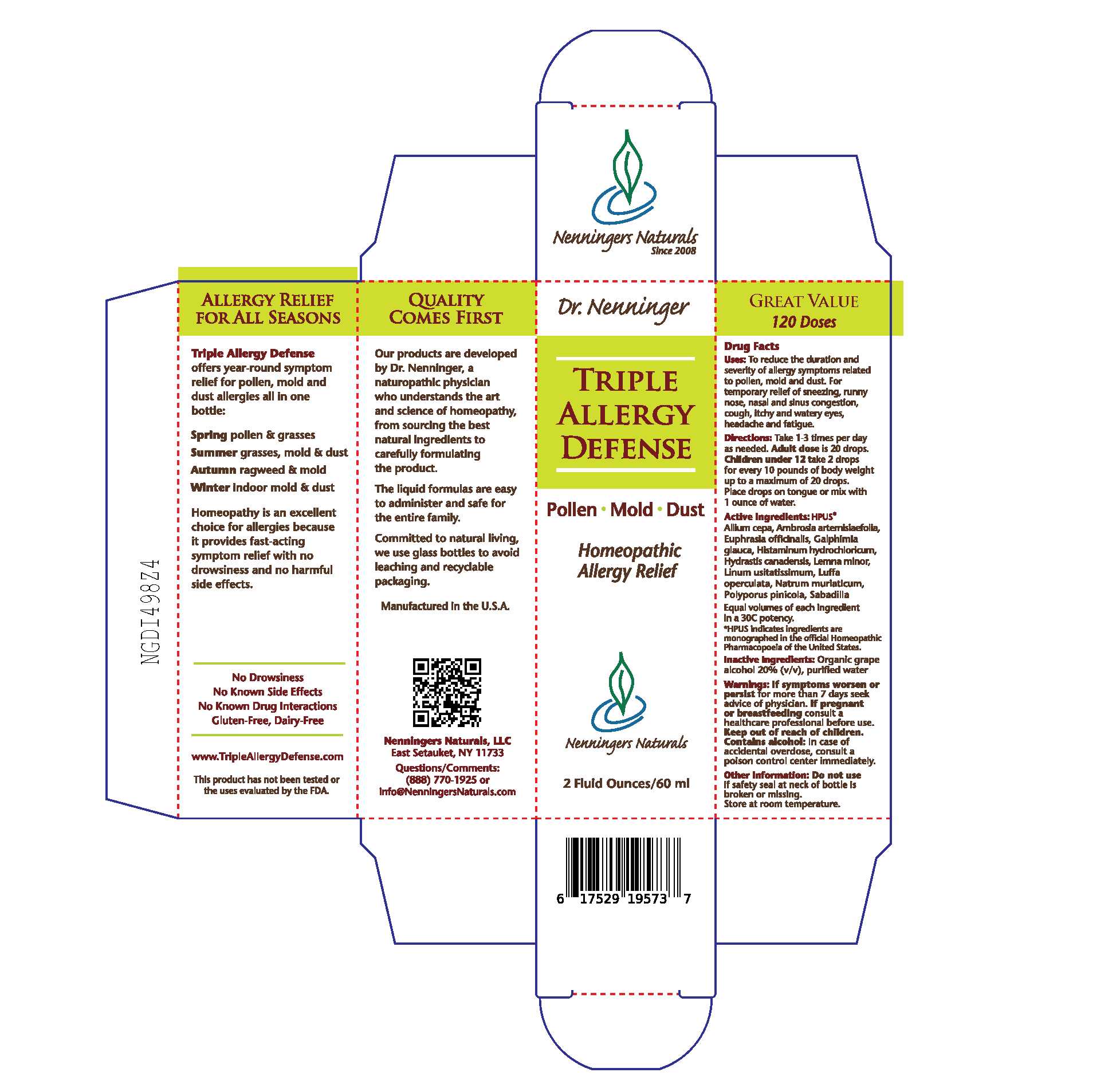
TRIPLE ALLERGY DEFENSE- allium cepa, ambrosia artemisiifolia, euphrasia officinalis, galphimia glauca, histaminum hydrochloricum, hydrastis canadensis, lemna minor, linum usitatissimum, luffa operculata, natrum muriaticum, polyporus pinicola, sabadilla liquid
Nenningers Naturals, LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Dr. Nenninger Triple Allergy Defense
Drug Facts
Uses: To reduce the duration and severity of allergy symptoms related to pollen, mold and dust. For temporary relief of sneezing, runny nose, nasal and sinus congestion, cough, itchy and watery eyes, headache and fatigue.
Directions: Take 1-3 times per day as needed. Adult dose is 20 drops. Children under 12 take 2 drops for every 10 pounds of body weight up to a maximum of 20 drops. Place drops on tongue or mix with 1 ounce of water.
Active Ingredients: HPUS*
Allium cepa, Ambrosia artemisiaefolia, Euphrasia officinalis, Galphimia glauca, Histaminum hydrochloricum, Hydrastis canadensis, Lemna minor, Linum usitatissimum, Luffa operculata, Natrum muriaticum, Polyporus pinicola, Sabadilla
Equal volumes of each ingredient in a 30C potency.
* HPUS indicates ingredients are monographed in the official Homeopathic Pharmacopeia of the United States
| TRIPLE ALLERGY DEFENSE
allium cepa, ambrosia artemisiifolia, euphrasia officinalis, galphimia glauca, histaminum hydrochloricum, hydrastis canadensis, lemna minor, linum usitatissimum, luffa operculata, natrum muriaticum, polyporus pinicola, sabadilla liquid |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Nenningers Naturals, LLC (967048666) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Medinatura New Mexico, inc. | 102783016 | manufacture(42731-032) | |