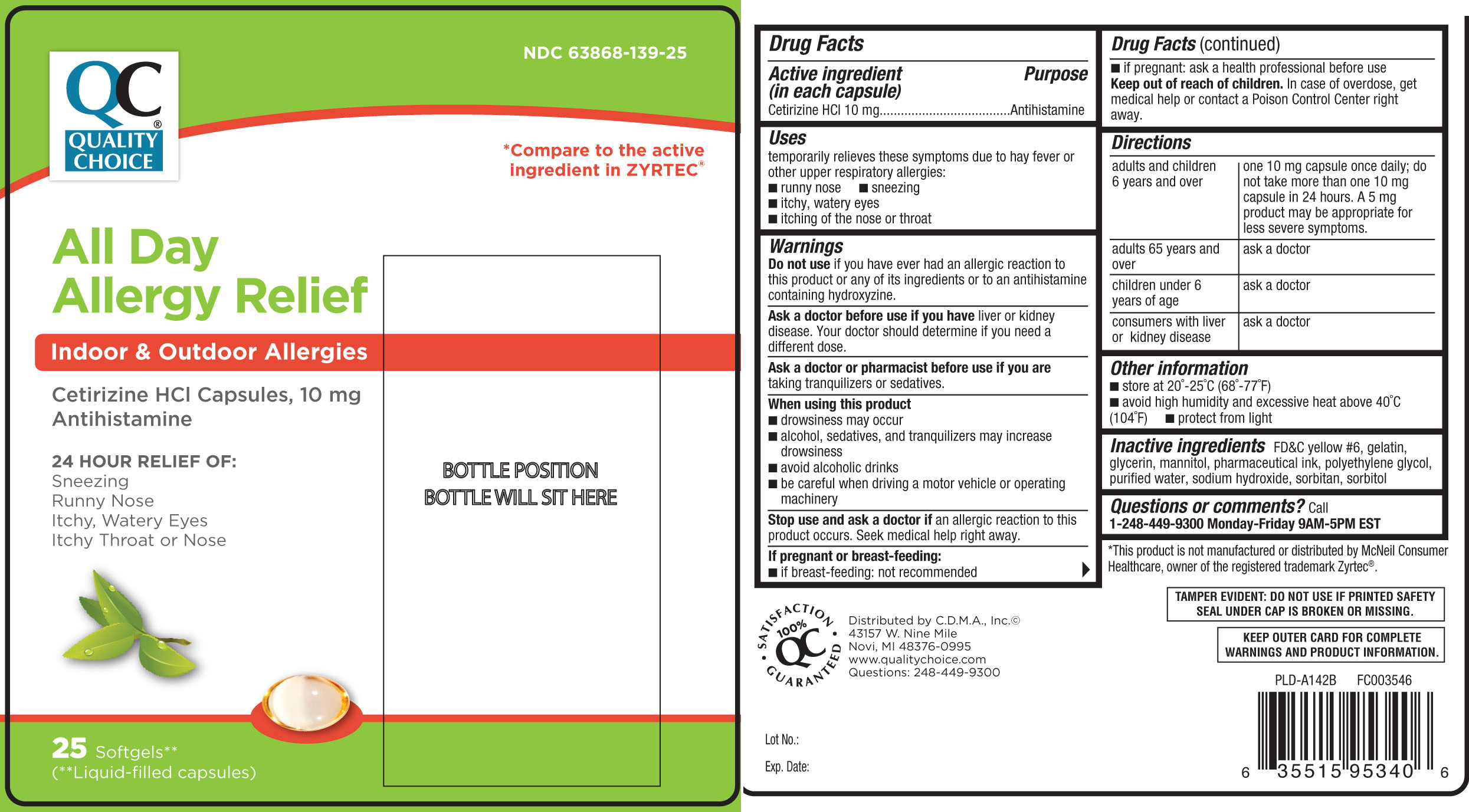
Label: ALL DAY ALLERGY- cetirizine hcl capsule
- NDC Code(s): 63868-139-25
- Packager: QUALITY CHOICE (Chain Drug Marketing Association)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 2, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each capsule)
- Purpose
- Uses
-
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
adults and children 6 years and over one 10 mg capsule once daily; do not take more than one 10 mg capsule in 24 hours. A 5 mg product may be appropriate for less severe symptoms. adults 65 years and over ask a doctor children under 6 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
*Compare to the active ingredient in ZYRTEC®
All Day Allergy Relief
Indoor & Outdoor Allergies
Cetirizine HCl Capsules, 10 mg
Antihistamine
24 Hour Relief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
SOFTGELS**
(**Liquid-filled capsules)
*This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Zyrtec®.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARD FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by C.D.M.A., Inc.©
43157 W. Nine Mile
Novi, MI 48376-0995
- Package Label
-
INGREDIENTS AND APPEARANCE
ALL DAY ALLERGY
cetirizine hcl capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-139 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) MANNITOL (UNII: 3OWL53L36A) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITAN (UNII: 6O92ICV9RU) SORBITOL (UNII: 506T60A25R) Product Characteristics Color yellow Score no score Shape OVAL Size 13mm Flavor Imprint Code CET Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-139-25 1 in 1 PACKAGE 04/30/2016 05/31/2024 1 25 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022429 04/30/2016 05/31/2024 Labeler - QUALITY CHOICE (Chain Drug Marketing Association) (011920774)