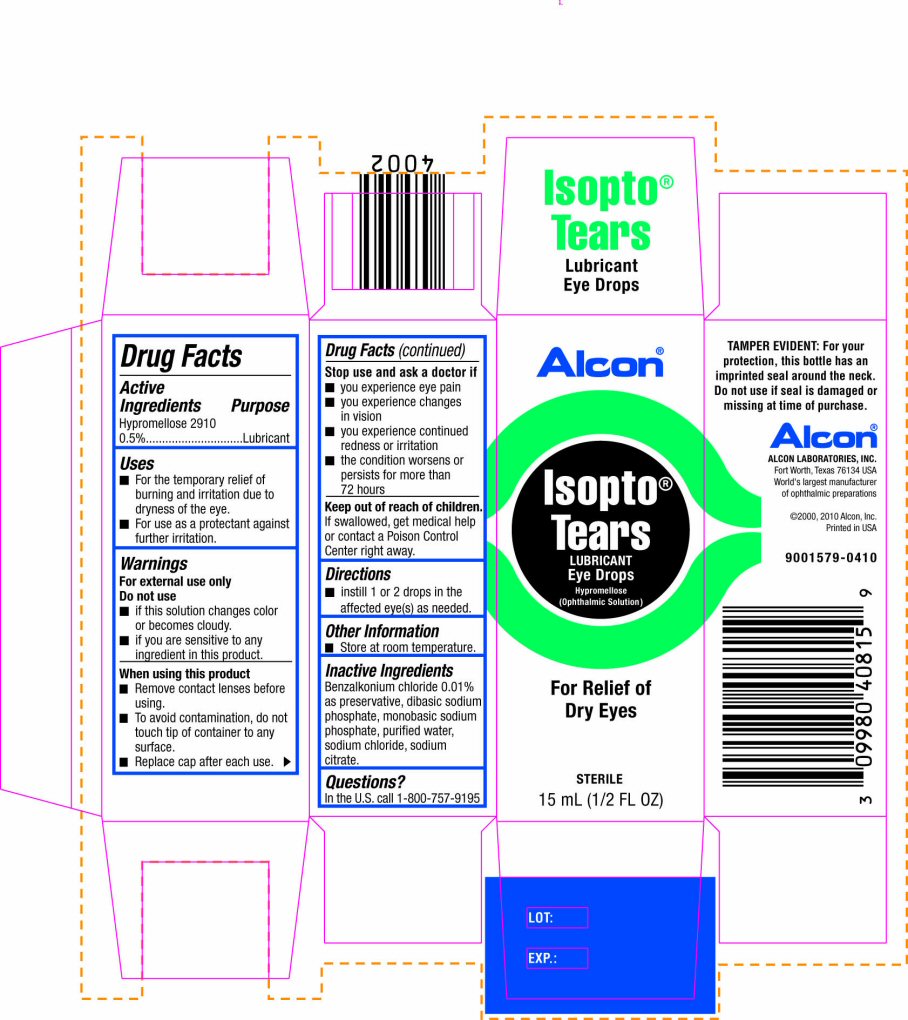
Label: ISOPTO TEARS- hypromellose 2910 solution
- NDC Code(s): 0998-0408-15
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- OTC - ACTIVE INGREDIENT SECTION
- OTC - PURPOSE SECTION
- INDICATIONS & USAGE SECTION
- WARNINGS SECTION
- OTC - WHEN USING SECTION
- OTC - STOP USE SECTION
- OTC - KEEP OUT OF REACH OF CHILDREN SECTION
- DOSAGE & ADMINISTRATION SECTION
- OTHER INFORMATION
- INACTIVE INGREDIENT SECTION
- OTC - QUESTIONS SECTION
-
PRINCIPAL DISPLAY PANEL
Alcon®
Isopto® Tears
LUBRICANT
Eye Drops
Hypromellose (Ophthalmic Solution)
For Relief of Dry Eyes
STERILE
15 mL (1/2 FL OZ)
TAMPER EVIDENT: For your protection, this bottle has an imprinted seal around the neck. Do not use if seal is damaged or missing at time of purchase.
Alcon®
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
World's largest manufacturer of ophthalmic preparations
©2000, 2010 Alcon, Inc.
Printed in USA
9001579-0410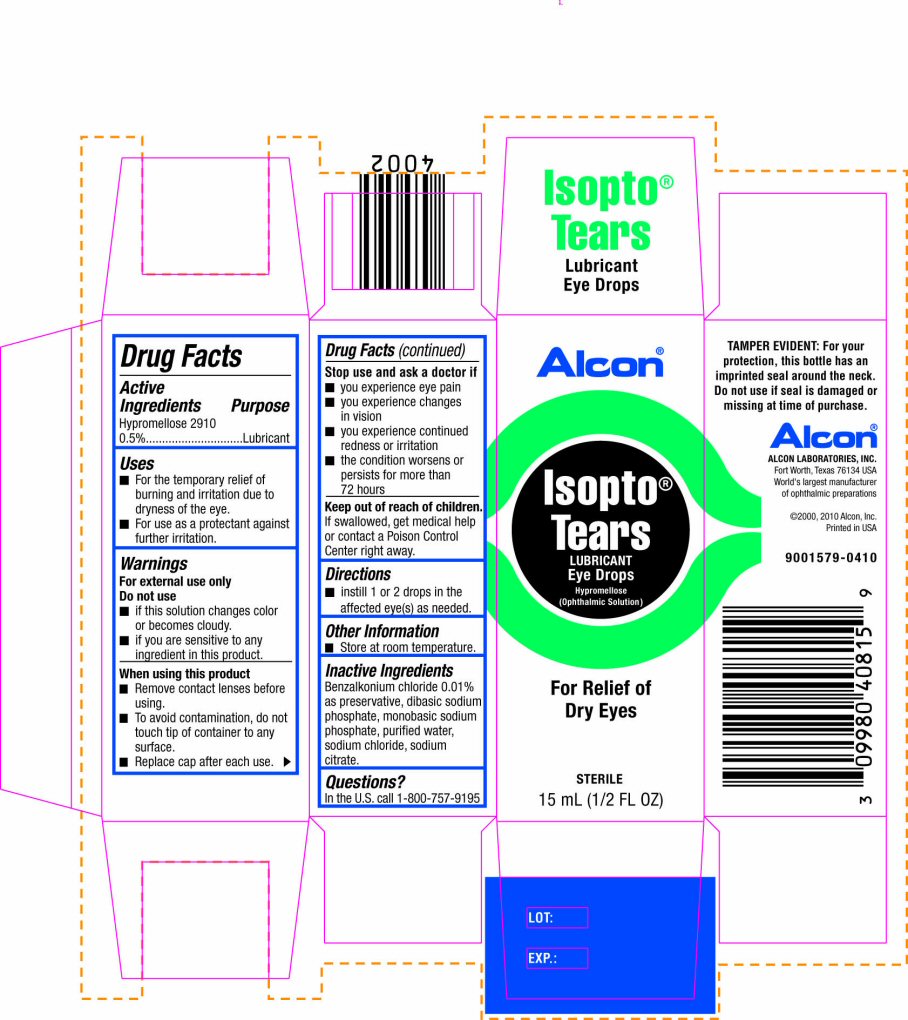
-
INGREDIENTS AND APPEARANCE
ISOPTO TEARS
hypromellose 2910 solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0998-0408 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hypromellose 2910 (4000 Mpa.s) (UNII: RN3152OP35) (Hypromellose 2910 (4000 Mpa.s) - UNII:RN3152OP35) Hypromellose 2910 (4000 Mpa.s) 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Sodium Phosphate, Dibasic (UNII: GR686LBA74) Sodium Phosphate, Monobasic (UNII: 3980JIH2SW) Water (UNII: 059QF0KO0R) Sodium Chloride (UNII: 451W47IQ8X) Sodium Citrate (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0998-0408-15 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 09/30/1990 12/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 09/30/1990 12/31/2024 Labeler - Alcon Laboratories, Inc. (008018525) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research Ltd 007672236 MANUFACTURE(0998-0408)