ACID REDUCER PLUS ANTACID- famotidine, calcium carbonate, magnesium hydroxide tablet, chewable
CVS Pharmacy
----------
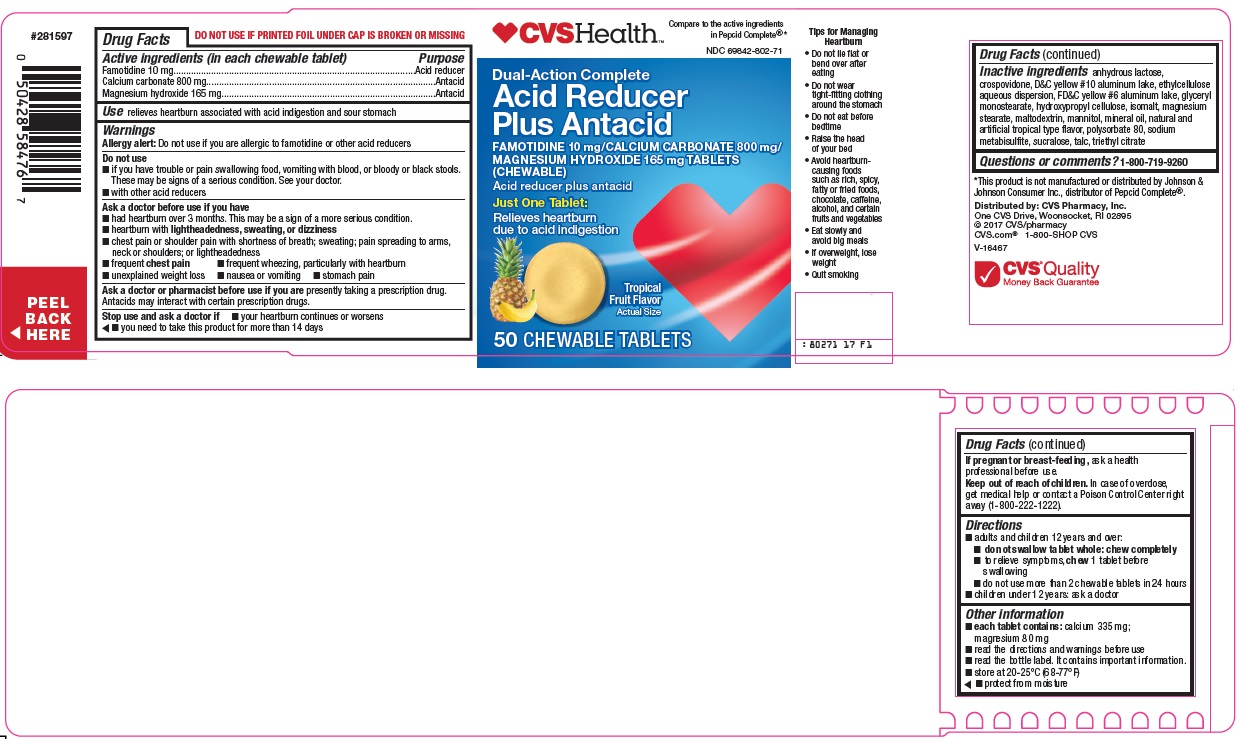
CVS Pharmacy, Inc. Acid Reducer Plus Antacid Drug Facts
Active ingredients (in each chewable tablet)
Famotidine 10 mg
Calcium carbonate 800 mg
Magnesium hydroxide 165 mg
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- •
- with other acid reducers
Ask a doctor before use if you have
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating, or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug. Antacids may interact with certain prescription drugs.
Directions
- •
- adults and children 12 years and over:
- •
- do not swallow tablet whole: chew completely
- •
- to relieve symptoms, chew 1 tablet before swallowing
- •
- do not use more than 2 chewable tablets in 24 hours
- •
- children under 12 years: ask a doctor
Other information
- •
- each tablet contains: calcium 335 mg; magnesium 80 mg
- •
- read the directions and warnings before use
- •
- read the bottle label. It contains important information.
- •
- store at 20-25°C (68-77°F)
- •
- protect from moisture
Inactive ingredients
anhydrous lactose, crospovidone, D&C yellow #10 aluminum lake, ethylcellulose aqueous dispersion, FD&C yellow #6 aluminum lake, glyceryl monostearate, hydroxypropyl cellulose, isomalt, magnesium stearate, maltodextrin, mannitol, mineral oil, natural and artificial tropical type flavor, polysorbate 80, sodium metabisulfite, sucralose, talc, triethyl citrate
Package/Label Principal Display Panel
CVS Health™
Compare to the active ingredients in Pepcid Complete®
Dual-Action Complete
Acid Reducer Plus Antacid
FAMOTIDINE 10 mg/CALCIUM CARBONATE 800 mg/MAGNESIUM HYDROXIDE 165 mg TABLETS (CHEWABLE)
Acid reducer plus antacid
Just One Tablet:
Relieves heartburn due to acid indigestion
Tropical Fruit Flavor
Actual Size
50 CHEWABLE TABLETS
| ACID REDUCER PLUS ANTACID
famotidine, calcium carbonate, magnesium hydroxide tablet, chewable |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |