XYLOCAINE - lidocaine hydrochloride injection, solution
Fresenius Kabi USA, LLC
----------
Xylocaine (lidocaine HCl Injection, USP) for Ventricular Arrhythmias
DESCRIPTION
Xylocaine (lidocaine HCl Injection, USP) is a sterile nonpyrogenic solution of an antiarrhythmic agent administered intravenously by direct injection.
Xylocaine Injection is composed of an aqueous solution of lidocaine hydrochloride. Lidocaine HCl (C14H22N2O•HCl) is chemically designated acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride and is represented by the following structural formula:
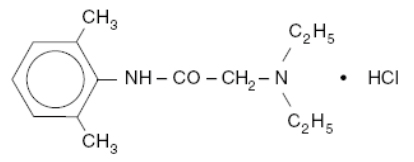
pH adjusted to 5.0 to 7.0 with sodium hydroxide and/or hydrochloric acid. Single use container. Solution does not contain preservatives.
CLINICAL PHARMACOLOGY
Mechanism of Action and Electrophysiology
Studies of the effects of therapeutic concentrations of lidocaine on the electrophysiological properties of mammalian Purkinje fibers have shown that lidocaine attenuates phase 4 diastolic depolarization, decreases automaticity, and causes a decrease or no change in excitability and membrane responsiveness. Action potential duration and effective refractory period of Purkinje fibers are decreased, while the ratio of effective refractory period to action potential is increased. Action potential duration and effective refractory period of ventricular muscle are also decreased. Effective refractory period of the AV node may increase, decrease or remain unchanged, and atrial effective refractory period is unchanged. Lidocaine raises the ventricular fibrillation threshold. No significant interactions between lidocaine and the autonomic nervous system have been described and consequently, lidocaine has little or no effect on autonomic tone.
Clinical electrophysiological studies with lidocaine have demonstrated no change in sinus node recovery time or sinoatrial conduction time. AV nodal conduction time is unchanged or shortened, and His-Purkinje conduction time is unchanged.
Hemodynamics
At therapeutic doses, lidocaine has minimal hemodynamic effects in normal subjects and in patients with heart disease. Lidocaine has been shown to cause no, or minimal, decrease in ventricular contractility, cardiac output, arterial pressure or heart rate.
Pharmacokinetics
Absorption, Distribution, and Excretion
After intravenous injection, lidocaine is quickly distributed in the tissues; the distribution volume (Vd = 1.7 L/kg) is decreased in patients with heart failure (Vd = 1 L/kg). Following intravenous administration of lidocaine the plasma concentration quickly decreases (half-life-α-phase [distribution] 8 min), the elimination half-life (β-phase) is 1.4 to 2.1 hours. In prolonged infusion (>24 h), plasma half-life clearly increases (3.2 h).
Plasma protein binding is about 70%. The plasma protein binding of lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 mcg free base per mL, 60 to 80 percent of lidocaine is protein bound. In addition to lidocaine concentration, the binding is dependent on the plasma concentration of the α-1-acid glycoprotein.
Therapeutic effects of lidocaine are generally associated with plasma levels of 6 to 25 μmole/L (1.5 to 6 mcg free base per mL). The blood to plasma distribution ratio is approximately 0.84. Objective adverse manifestations become increasingly apparent with increasing plasma levels above 6 mcg free base per mL. Lidocaine readily crosses the placental and blood-brain barriers.
Metabolism
Lidocaine is rapidly metabolized by the liver, and less than 10% of a dose is excreted unchanged in the urine. A major pathway of metabolism is through oxidative N-dealkylation to yield the active metabolites monoethylglycinexylidide (MEGX) and glycinexylidide (GX). The pharmacological/toxicological activities of these metabolites are similar to, but less potent than, lidocaine. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6-dimethylaniline.
In vitro studies with human liver microsomes and recombinant human cytochrome P450 (CYP) isoforms showed that both CYP1A2 and CYP3A4 enzymes are the major CYP isoforms involved in lidocaine N-dealkylation.
Drug Interactions
Cimetidine: Cimetidine reduces liver blood flow and thus systemic clearance of drugs that are highly extracted by the liver. Clinical Experiments showed that the concomitant administration of cimetidine reduces the systemic clearance of lidocaine and increases lidocaine serum concentration by as much as 50%. Thus, therapeutic serum levels of lidocaine may rise to toxic levels when cimetidine is used concomitantly. Ranitidine has not displayed such an effect.
Propranolol: Administration of propranolol during infusion of lidocaine may increase the plasma concentration of lidocaine by about 30%. Patients already receiving propranolol tend to have higher lidocaine levels than controls.
Amiodarone: Lidocaine area under the curve (AUC) is increased when amiodarone is administered but that of N-monodesethylated lidocaine is decreased. Moreover, the systemic clearance of lidocaine is decreased, while the elimination half-life (t½) and the distribution volume at steady state of lidocaine remained unchanged. The interaction between amiodarone and lidocaine may be explained by the inhibition of CYP3A4 by amiodarone and/or by its main metabolite N-monodesethylarniodarone (DEA).
Special Populations
Renal Insufficiency: Renal dysfunction does not affect lidocaine kinetics, buy may increase the accumulation of metabolites. While renal excretion accounts for only a small fraction of the total clearance of lidocaine, approximately 50% of the total elimination of the active metabolite glycinexylidide (GX) is through renal excretion. Patients with significant renal disease may be at risk of accumulating GX after prolonged infusion, which could contribute to the development of CNS toxicity.
Dialysis has negligible effects on the kinetics of lidocaine.
Liver Impairment: Because of the rapid rate at which lidocaine is metabolized, liver disease and conditions associated with reduced hepatic blood flow (i.e., heart failure, shock, treatment with beta-adrenoceptor blocking drugs) may alter lidocaine kinetics. Considerable prolongation is to be expected in the half-life of lidocaine in patients with liver dysfunction, half-life may be two-fold or greater (5 h).
Geriatrics: The pharmacokinetics of Xylocaine Injection have not been formally studied in patients 65 years of age. However, published data show that lidocaine has been used in patients 65 years and older. After administration of usual dosages, elderly patients may be more susceptible to lidocaine adverse events because of higher systemic drug exposure. The disposition of lidocaine may be altered in elderly patients with or without concurrent disease. After intravenous or epidural administrations, elderly patients may show reduced plasma clearance, prolonged elimination half-life and increased volume of distribution compared to younger controls. Reductions in plasma clearance may be greater in elderly male patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
INDICATIONS AND USAGE
Xylocaine administered intravenously is specifically indicated in the acute management of ventricular arrhythmias such as those occurring in relation to acute myocardial infarction, or during cardiac manipulation, such as cardiac surgery.
CONTRAINDICATIONS
Xylocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type. Xylocaine should not be used in patients with Stokes-Adams syndrome, Wolff-Parkinson-White syndrome, or with severe degrees of sinoatrial, atrioventricular, or intraventricular block in the absence of an artificial pacemaker.
WARNINGS
IN ORDER TO MANAGE POSSIBLE ADVERSE REACTIONS, RESUSCITATIVE EQUIPMENT, OXYGEN AND OTHER RESUSCITATIVE DRUGS SHOULD BE IMMEDIATELY AVAILABLE WHEN XYLOCAINE (LIDOCAINE HCl INJECTION, USP) IS USED.
Systemic toxicity may result in manifestations of central nervous system depression (sedation) or irritability (twitching), which may progress to frank convulsions accompanied by respiratory depression and/or arrest. Early recognition of premonitory signs, assurance of adequate oxygenation and, where necessary, establishment of artificial airway with ventilatory support are essential to management of this problem. Should convulsions persist despite ventilatory therapy with oxygen, small increments of anticonvulsant drugs may be used intravenously. Examples of such agents include benzodiazepines (eg, diazepam), ultrashort-acting barbiturates (eg, thiopental or thiamylal), or a short-acting barbiturate (eg, pentobarbital or secobarbital). If the patient is under anesthesia, a short-acting muscle relaxant (eg, succinylcholine) may be used. Longer-acting drugs should be used only when recurrent convulsions are evidenced.
Should circulatory depression occur, vasopressors may be used.
Constant electrocardiographic monitoring is essential to the proper administration of Xylocaine. Signs of excessive depression of cardiac electrical activity such as sinus node dysfunction, prolongation of the P-R interval and QRS complex or the appearance or aggravation of arrhythmias, should be followed by flow adjustment and, if necessary, prompt cessation of the intravenous infusion of this agent. Occasionally, acceleration of ventricular rate may occur when Xylocaine is administered to patients with atrial flutter or fibrillation.
PRECAUTIONS
General
Caution should be employed in the use of Xylocaine in patients with severe liver or kidney disease because accumulation of the drug or metabolites may occur.
Xylocaine should be used with caution in the treatment of patients with hypovolemia, severe congestive heart failure, shock, and all forms of heart block. In patients with sinus bradycardia or incomplete heart block, the administration of Xylocaine intravenously for the elimination of ventricular ectopic beats, without prior acceleration in heart rate (eg, by atropine, isoproterenol or electric pacing), may promote more frequent and serious ventricular arrhythmias or complete heart block (see CONTRAINDICATIONS).
Dosage should be reduced for pediatric patients and for debilitated and/or elderly patients, commensurate with their age and physical status.
The safety of amide local anesthetic agents in patients with genetic predisposition to malignant hyperthermia has not been fully assessed; therefore, lidocaine should be used with caution in such patients.
In hospital environments where drugs known to be triggering agents for malignant hyperthermia (fulminant hypermetabolism) are administered, it is suggested that a standard protocol for management should be available.
It is not known whether lidocaine may trigger this reaction; however, large doses resulting in significant plasma concentrations, as may be achieved by intravenous infusion, pose potential risk to these individuals. Recognition of early unexplained signs of tachycardia, tachypnea, labile blood pressure and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the triggering agent and institution of treatment including oxygen therapy, supportive measures and dantrolene (for details see dantrolene package insert).
Information for Patients
The patient should be advised of the possible occurrence of the experiences listed under ADVERSE REACTIONS.
Drug Interactions
Xylocaine should be used with caution in patients with digitalis toxicity accompanied by atrioventricular block. Concomitant use of beta-blocking agents or cimetidine may reduce hepatic blood flow and thereby reduce lidocaine clearance.
Lidocaine and tocainide are pharmacodynamically similar. The concomitant use of these two agents may cause an increased incidence of adverse reactions, including central nervous system adverse reactions such as seizure.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility of Xylocaine have not been conducted.
Pregnancy: Teratogenic Effects
Pregnancy Category B.
Reproduction studies have been performed in rats at doses up to 6.6 times the maximum human doses and have revealed no significant findings. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery
The effects of Xylocaine on the mother and the fetus, when used in the management of cardiac arrhythmias during labor and delivery, are not known. Lidocaine readily crosses the placental barrier.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when lidocaine is administered to a nursing woman.
Pediatric Use
Controlled clinical studies have not been conducted in the pediatric population to establish safety and efficacy in this population (see DOSAGE AND ADMINISTRATION).
Geriatric
Clinical studies of Xylocaine Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Patients with significant renal disease may be at risk of accumulating the active metabolite GX after prolonged infusion, which could contribute to the development of CNS toxicity. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see CLINICAL PHARMACOLOGY, Geriatrics).
ADVERSE REACTIONS
Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. Adverse experiences may result from high plasma levels caused by excessive dosage or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported. The adverse experiences under Central Nervous System and Cardiovascular System are listed, in general, in a progression from mild to severe.
Central Nervous System
CNS reactions are excitatory and/or depressant, and may be characterized by light-headedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory reactions may be very brief or may not occur at all, in which case, the first manifestation of toxicity may be drowsiness, merging into unconsciousness and respiratory arrest.
Cardiovascular System
Cardiovascular reactions are usually depressant in nature and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic reactions as a result of sensitivity to lidocaine are extremely rare and, if they occur, should be managed by conventional means.
DRUG ABUSE AND DEPENDENCE
Although specific studies have not been conducted, Xylocaine has been used clinically without evidence of abuse of this drug or of psychological or physical dependence as a result of its use.
OVERDOSAGE
Overdosage of Xylocaine (lidocaine HCl Injection, USP) usually results in signs of central nervous system or cardiovascular toxicity (see ADVERSE REACTIONS).
Should convulsions or signs of respiratory depression and arrest develop, the patency of the airway and adequacy of ventilation must be assured immediately. Should convulsions persist despite ventilatory therapy with oxygen, small increments of anticonvulsive agents may be given intravenously. Examples of such agents include a benzodiazepine (eg, diazepam), an ultrashort-acting barbiturate (eg, thiopental or thiamylal), or a short-acting barbiturate (eg, pentobarbital or secobarbital). If the patient is under general anesthesia, a short-acting muscle relaxant (eg, succinylcholine) may be administered.
Should circulatory depression occur, vasopressors may be used. Should cardiac arrest occur, standard CPR procedures should be instituted.
Dialysis is of negligible value in the treatment of acute overdosage from Xylocaine.
DOSAGE AND ADMINISTRATION
Adults
Single Direct Intravenous Injection (bolus)
The usual dose is 50 to 100 mg of lidocaine hydrochloride (0.7 to 1.4 mg/kg; 0.32 to 0.63 mg/lb) administered intravenously under ECG monitoring. This dose may be administered at the rate of approximately 25 to 50 mg/min (0.35 to 0.7 mg/kg/min; 0.16 to 0.32 mg/lb/min). Sufficient time should be allowed to enable a slow circulation to carry the drug to the site of action. If the initial injection of 50 to 100 mg does not produce a desired response, a second dose may be injected after 5 minutes. NO MORE THAN 200 TO 300 mg OF LIDOCAINE HYDROCHLORIDE SHOULD BE ADMINISTERED DURING A ONE HOUR PERIOD.
Continuous Intravenous Infusion
Following bolus administration, intravenous infusions of Xylocaine may be initiated at the rate of 1 to 4 mg/min of lidocaine hydrochloride (0.014 to 0.057 mg/kg/min; 0.006 to 0.026 mg/lb/min). The rate of intravenous infusions should be reassessed as soon as the patient’s basic cardiac rhythm appears to be stable or at the earliest signs of toxicity. It should rarely be necessary to continue intravenous infusions of lidocaine for prolonged periods.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit. Do not use if solution is discolored or cloudy.
HOW SUPPLIED
For direct intravenous injection, Xylocaine (lidocaine HCl Injection, USP) without preservatives is supplied in the following dosage form:
| Product No. | NDC No. | Strength
| |
| 491889 | 63323-498-89 | 20 mg/mL | 5 mL ampules packaged in tens. |
Solution should be stored at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
XYLOCAINE is a trademark of APP Pharmaceuticals, LLC.
PACKAGE LABEL - PRINCIPAL DISPLAY - Xylocaine® 5 mL Ampule Label
NDC 63323-498-89
491889
Xylocaine® (lidocaine HCl) Solution
100 mg (20 mg/mL)
IV Intravenous Injection for Ventricular Arrhythmias
5 mL ampule
Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Xylocaine® 5 mL Carton Panel
NDC 63323-498-89
491889
Xylocaine® (lidocaine HCl) Solution
100 mg (20 mg/mL)

| XYLOCAINE
lidocaine hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Fresenius Kabi USA, LLC (608775388) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fresenius Kabi Austria GmbH | 300206604 | ANALYSIS(63323-498) , MANUFACTURE(63323-498) | |
