Label: ALCOHOL FREE HAND SANITIZER- benzalkonium chloride liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 79993-110-01, 79993-110-02, 79993-110-05, 79993-110-16 - Packager: Devbond Consulting, L.l.c
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 5, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
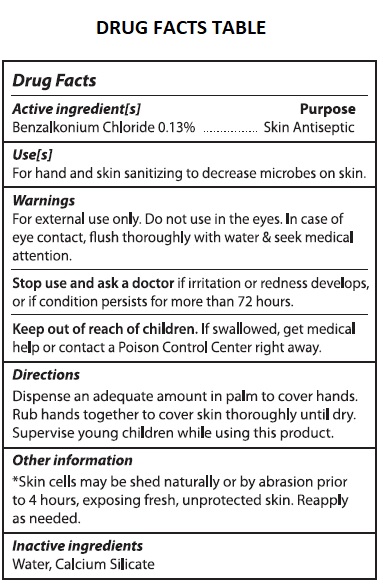
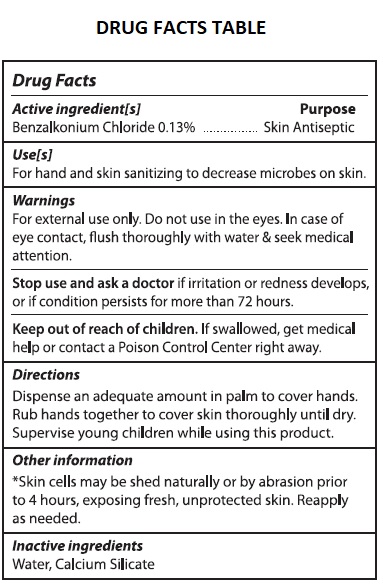
- Drug Facts
- Active ingredients[s]
- Purpose
- Use[s]
- Warnings
- Directions
- Other information
- Inactive ingredients
-
SPL UNCLASSIFIED SECTION
Kills 99.99% of Bacteria & Viruses
By DevbondUSA
4-HOUR HAND SANITIZER
NON-FLAMMABLE
NON-TOXIC
DYE FREE
FRAGRANCE FREE
UltraNanoClean Hand Sanitizer has proven residual antimicrobial benefits for up to four hours. It's active ingredient is a preferred skin antiseptic that kills germs on contacts and inhibits future microbial growth. In addition, the proprietary mineral blend enhances product bonding and long lasting effectiveness.
• Kills 99.9% of illness-causing germs on contact & persists on skin for up to 4 hours
• Alcohol Free, Foaming Formula won't dry hands, sting or cause cracking
• Moisture-infused foam leaves skin feeling nourished & silky, even after repeated applications
• Outperforms alcohol-based sanitizers by persisting on skin; guarding against exposure
MADE IN THE USA
Distributed by:
DevBond USA
1836 Augusta Drive
Suite 16
Houston TX 77057
(713) 256-6160
FOR EXTERNAL USE ONLY
FDA Registered
- Packaging
-
INGREDIENTS AND APPEARANCE
ALCOHOL FREE HAND SANITIZER
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79993-110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CALCIUM SILICATE (UNII: S4255P4G5M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79993-110-02 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2021 2 NDC:79993-110-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/05/2021 3 NDC:79993-110-01 3785 mL in 1 JUG; Type 0: Not a Combination Product 08/01/2020 4 NDC:79993-110-05 18927 mL in 1 PAIL; Type 0: Not a Combination Product 08/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/01/2020 Labeler - Devbond Consulting, L.l.c (023344086)