EVITHROM- human thrombin liquid
Ethicon Inc
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EVITHROM safely and effectively. See full prescribing information for EVITHROM.
EVITHROM [thrombin, topical (human) Frozen solution for topical use Initial U.S. Approval: 2007 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
The potency expressed in units is determined using a clotting assay against an internal reference standard for potency that has been calibrated against the World Health Organisation (WHO) Second International Standard for Thrombin, 01/580. Therefore, a unit used herein is equivalent to an International Unit. CONTRAINDICATIONSWARNINGS AND PRECAUTIONSADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact ETHICON Customer Support Center at (877) 384-4266 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 6/2020 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
EVITHROM® Thrombin, Topical (Human) is indicated as an aid to hemostasis whenever oozing blood and minor bleeding from capillaries and small venules is accessible and control of bleeding by standard surgical techniques (such as suture, ligature or cautery) is ineffective or impractical.
EVITHROM® Thrombin, Topical (Human) may be used in conjunction with an Absorbable Gelatin Sponge, USP.
2 DOSAGE AND ADMINISTRATION
For topical use only. DO NOT INJECT.
2.1 Dose
The amount of EVITHROM® required depends upon the area of tissue to be treated and the method of application. As an approximate guide, volumes up to 10 mL were used in clinical studies where EVITHROM® was applied in conjunction with Absorbable Gelatin Sponge, USP.
2.2 Preparation
Thaw prior to use
Thaw EVITHROM® in one of the following ways:
- 2°C to 8°C (refrigerator): vials thaw within 1 day; or
- 20°C to 25°C (room temperature): vials thaw within 1 hour; or
- 37°C for 2 ml and 5 ml vials only: vials thaw within 10 minutes. Do not exceed 37°C and do not leave vials at 37°C for longer than 10 minutes.
Once thawed
Remove the flip-off plastic cap from the vial to expose the rubber stopper.
If using EVITHROM® alone: withdraw the thrombin solution from the glass vial with a sterile needle and syringe.
If using EVITHROM® in conjunction with an absorbable gelatin sponge: remove the rubber stopper (by removing the metal pull tab) and transfer thrombin solution into a sterile container large enough to immerse sponge
2.3 Administration
DO NOT INJECT. Apply only on the surface of bleeding tissue.
EVITHROM® alone
- Sponge target surface (do not wipe) or suction free of blood before application.
- Flood the surface with EVITHROM® using a sterile syringe and small gauge needle.
- After treatment, avoid sponging the clot to assure that it remains securely in place.
EVITHROM® in conjunction with absorbable gelatin sponge, USP
- Using aseptic techniques, immerse gelatin sponge of desired shape into the sterile container with the EVITHROM® solution.
- Vigorously knead the sponge with moistened gloved fingers until all air is expelled and it can return to its original size and shape.
- Hold the saturated sponge in place with gauze or cotton pledget using moderate pressure until hemostasis is achieved.
Vials are for single use only. Discard unused contents.
3 DOSAGE FORMS AND STRENGTHS
EVITHROM® is supplied as a frozen solution in the following packages:
- Vial containing 2 ml, 5 ml or 20 ml. Each vial contains 800-1200 units/ml of Thrombin, Topical (Human).
The potency expressed in units is determined using a clotting assay against an internal reference standard for potency that has been calibrated against the World Health Organisation (WHO) Second International Standard for Thrombin, 01/580. Therefore, a unit used herein is equivalent to an International Unit.
4 CONTRAINDICATIONS
- Do not use in individuals known to have anaphylactic or severe systemic reaction to human blood products.
- Do not use for the treatment of severe or brisk arterial bleeding.
5 WARNINGS AND PRECAUTIONS
5.2 Transmission of Infectious Agents
Because this product is made from human plasma, it may carry a risk of transmitting infectious agents, such as viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk of transmitting an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections and by inactivating and removing certain viruses.
Despite these measures, such products can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in such products. The physician should discuss the risks and benefits of this product with the patient.
6 ADVERSE REACTIONS
The most common adverse reactions during clinical trials (reported in at least 2% of subjects treated with EVITHROM®) were prolonged activated partial thromboplastin time, increased INR, decreased lymphocyte count, prolonged prothrombin time and increased neutrophil count.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug product cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Anaphylactic reactions may occur in rare cases. No adverse events of this type were reported during the conduct of the clinical trials. Mild reactions can be managed with anti-histamines. Severe hypotensive reactions require immediate intervention using current principles of shock therapy.
In a phase III multicenter, prospective, controlled, randomized, double-blinded study of 305 subjects where EVITHROM® (n=153) was compared with bovine thrombin (n=152), occurrence of adverse events was not statistically different between the two groups.
Overall, adverse events occurred in similar proportions of subjects in the two study groups (see Table 1). No clinically significant differences were seen in age (<65 years, >65 years) or gender subgroup analyses of adverse events.
At least one serious adverse event (SAE) was reported for 26/153 (17%) subjects treated with human thrombin and 17/152 (11%) subjects treated with bovine thrombin. The SAEs reported were associated with post-surgical complications (e.g. wound infection 3/153 for EVITHROM® and 2/152 for bovine thrombin) and the medical condition of the subject and were not considered related to study drug. Two subjects (1.3%) in EVITHROM® group experienced a treatment emergent severe adverse event: respiratory arrest and post-procedural hematoma (in one subject) and extradural hematoma. Three subjects in the bovine thrombin group experienced a treatment emergent severe adverse event: hyperhidrosis, pyrexia and post-procedural hematoma. No deaths were reported during the study period.
Viral serology was not monitored during the study with EVITHROM®. However, no adverse events indicative of infection with transfusion-transmissible agents were reported.
| Thrombin Type | |||
|---|---|---|---|
| System Organ Class/Adverse Event | EVITHROM®
(n=153) | Bovine (n=152) | Total (n=305) |
| Investigations | 11 (7.2%) | 14 (9.2%) | 25 (8.2%) |
| Activated partial thromboplastin time increased | 4 (2.6%) | 8 (5.3%) | 12 (3.9%) |
| International normalized ratio increased | 4 (2.6%) | 5 (3.3%) | 9 (3.0%) |
| Lymphocyte count decreased | 4 (2.6%) | 2 (1.3%) | 6 (2.0%) |
| Prothrombin time prolonged | 4 (2.6%) | 8 (5.3%) | 12 (3.9%) |
| Neutrophil count increased | 3 (2.0%) | 2 (1.3%) | 5 (1.6%) |
| Skin and Subcutaneous Tissue Disorders | 1 (0.7%) | 3 (2.0%) | 4 (1.3%) |
| Pruritis | 1 (0.7%) | 3 (2.0%) | 4 (1.3%) |
| General Disorders and Administration Site Conditions | 0 | 3 (2.0%) | 3 (1.0%) |
Immunogenicity
In the clinical study, serum samples were collected at baseline and at 5 weeks post-surgery for evaluation of antibodies to bovine thrombin, bovine Factor V/Va, human thrombin, and human Factor V/Va. Samples were collected at both time points for 81.3% of the subjects. The ELISA data were adjudicated by a panel of experts blinded to treatment assignment. After reviewing all data, the panel used an algorithm for assigning outcomes for each antigen: seroconversion negative or seroconversion positive.
The protocol did not specify any comparative analysis for immunogenicity data, only descriptive statistics. The adjudicated results show that 3.3% of the subjects treated with EVITHROM® developed antibodies to any of the four antigens, compared to 12.7% of the subjects developing antibodies in the control group (bovine thrombin). 7.94% of the subjects treated with bovine thrombin (control group) developed antibodies to bovine thrombin and 9.52% of these subjects developed antibodies to bovine Factor V/Va. A few control subjects had antibodies that cross-reacted with human thrombin, but none had antibodies that cross-reacted with human Factor V/Va. None of the patients treated with EVITHROM® developed detectable antibodies to human thrombin or to human Factor V/Va.
The detection of antibody formation is highly dependent upon the sensitivity and specificity of the assay. The observed incidence of a positive signal in an assay may be influenced by several factors including timing of sampling, sample handling, concomitant medications, or underlying disease. Therefore, direct comparison of incidence of antibody development to human thrombin, bovine thrombin, human Factor V/Va or bovine Factor V/Va following administration of EVITHROM® with incidence of antibody development following administration of other products may be misleading and the clinical significance of these findings is unknown.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no clinical data with EVITHROM® use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with EVITHROM. It is not known whether EVITHROM® can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of EVITHROM® in human milk, the effect on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EVITHROM® and any potential adverse effects on the breastfed infant from EVITHROM® or from the underlying maternal condition.
8.4 Pediatric Use
Of the 155 patients undergoing liver surgery who were treated in adequate and well controlled clinical studies of EVICEL® Fibrin Sealant (Human), in which EVITHROM® is a component, eight were pediatric patients. Of these, five were less than 2 years old and three were between 2 and 12 years old. Use of EVITHROM® in pediatric patients is supported by these data and by extrapolation of findings for safety and efficacy in adults.
11 DESCRIPTION
EVITHROM® Thrombin, Topical (Human) is a sterile solution, pH 6.8-7.2, containing purified human thrombin for the activation of clotting.
Frozen EVITHROM® consists of a white to slightly yellowish opaque mass. When thawed, EVITHROM® is clear to slightly opalescent and colorless to slightly yellowish.
The composition of EVITHROM® is as follows:
Active Ingredients:
Human thrombin (800-1200 units/ml)
Other Ingredients:
Calcium chloride, Human albumin, Mannitol, Sodium acetate, Water for injection (WFI).
EVITHROM® is made from pooled Human Source and Recovered Plasma obtained from US licensed plasma collection centers.
Individual plasma units obtained for production of EVITHROM® are tested by licensed serological tests for HBsAg, HIV 1 & 2 Ab and HCV Ab and recovered plasma units are also tested for HTLV I/II. Additionally, the plasma units are tested by licensed Nucleic Acid Testing (NAT) for HIV-1, HCV, HBV, HAV and parvovirus 19. All tests for HIV, HCV, HBV and HAV must be negative (non-reactive). However, since the effectiveness of the HBV and HAV NAT methods in detecting low levels of viral material is still under investigation, the significance of a negative result for these viruses is unknown. The level of parvovirus B19 contamination is not permitted to exceed 10,000 copies/ml. This limit is applied to restrict the viral load of parvovirus B19 in the starting plasma pool. In addition to the screening of plasma units, each manufacturing pool is tested for HBsAg, HIV-1 & 2 Ab, HCV NAT and for Parvovirus B19 by NAT. Manufacturing pool testing, however, is of lower sensitivity than individual unit testing.
EVITHROM® is manufactured by chromatographic purification of prothrombin from cryo-poor plasma followed by activation with calcium chloride. The manufacturing process includes two targeted steps for inactivation or removal of viruses. The first of these is treatment with a solvent/detergent (S/D) mixture (1% tri-n-butyl phosphate, 1% Triton X-100) for 6 hours at 26°C to inactivate lipid enveloped viruses.
The S/D reagents are removed by cation exchange chromatography. Mannitol and human albumin are used to stabilize the solution, which undergoes nanofiltration for removal of both enveloped and non-enveloped viruses. After nanofiltration, the solution is formulated with calcium chloride, sterile filtered and aseptically filled and frozen.
The effectiveness of the S/D treatment and nanofiltration procedures for reducing virus content has been assessed using a series of viruses with a range of physico-chemical characteristics. The results of the validation studies are summarized in Table 2:
| Virus | HIV-1 | SBV | BVDV | PRV | EMCV | HAV | MVM |
|---|---|---|---|---|---|---|---|
| Reduction factor (log10) | |||||||
| HIV-1: Human Immunodeficiency Virus Type 1 | |||||||
| SBV: Sindbis Virus | |||||||
| BVDV: Bovine Viral Diarrhea Virus | |||||||
| PRV: Pseudorabies Virus | |||||||
| EMCV: Encephalomyocarditis virus | |||||||
| HAV: Hepatitis A Virus | |||||||
| MVM: Minute Virus of Mouse | |||||||
| SD Treatment | >5.8 | >5.0 | >4.6 | >4.2 | Not Done | Not Done | Not Done |
| Nanofiltration | >4.6 | Not Done | >5.6 | >5.7 | >7.4 | >7.5 | >6.3 |
| Global Reduction Factor | >10.4 | >5.0 | >10.2 | >9.9 | >7.4 | >7.5 | >6.3 |
12 CLINICAL PHARMACOLOGY
EVITHROM® requires no intermediate physiological agent because it clots the fibrinogen of the blood directly. Failure to clot blood occurs in the rare case where the primary clotting defect is the absence of fibrinogen itself. The speed with which thrombin clots blood is dependent upon the concentration of both thrombin and fibrinogen.
12.1 Mechanism of Action
Thrombin (coagulation factor IIa) is a highly specific protease that transforms plasma fibrinogen into fibrin which, in the presence of Factor XIII in the patient's plasma, is crosslinked to form a stable clot. When applied to a surgical wound where bleeding is present, thrombin activates fibrinogen in the patient's plasma to form fibrin, which results in clot formation and hemostasis. The fibrin clot is stabilized by cross-linking occurring as a result of activation of the patient's endogenous factor XIII, which requires the presence of calcium.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of EVITHROM® due to the human origin of thrombin.
Studies were performed in bacteria to determine mutagenicity of human thrombin alone, and solvent/detergent residues [tri-n-butyl phosphate (TnBP) and Triton X-100], used in the virus inactivation manufacturing step. These studies were negative for both Thrombin and for TnBP or Triton X-100 at all concentrations tested. All concentrations of the combination of TnBP and Triton X-100 also tested negative in assays performed to determine mammalian cell mutagenicity, chromosomal aberrations and micronuclei induction.
The effect of EVITHROM® on fertility has not been evaluated. Reproductive studies were performed in rats with the combination of solvent detergent impurities, TnBP and Triton X-100 at doses up to approximately 600-fold human dose of TnBP (900 µg/kg/day) and 3000-fold human dose of Triton X-100 (4500 μg/kg/day) resulted in increased post-implantation loss and an increased number of late resorptions. Other studies performed with combinations of TnBP (300-fold human dose, 450 µg/kg/day) and Triton X-100 (1500-fold human dose, 2250 µg/kg/day) resulted in increased resorption rates, decreased fetal body weights, and an increased number of runts. No embryo-fetal adverse effects were observed at doses up to 300 µg/kg/day TnBP and 1500 µg/kg/day Triton X-100, 200-fold and 1000-fold the human dose, respectively.
13.2 Animal Toxicology and/or Pharmacology
EVICEL® Fibrin Sealant (Human), which includes EVITHROM® as one of the active components, was classified as non-irritant in the Primary Cutaneous Irritation Test and slightly irritant in the Ocular Irritation test.
Neurotoxicity studies performed with EVITHROM® or with EVICEL® confirmed that intracerebral application of thrombin was not associated with any evidence of neurotoxicity. No toxicological effects due to solvent/detergent reagents [tri-n-butyl phosphate (TnBP) and Triton X-100] used in the virus inactivation procedure are expected since the residual levels are less than 5 µg/ml.
14 CLINICAL STUDIES
EVITHROM® was compared with bovine thrombin in a phase III multicenter, prospective, randomized, controlled, double-blinded study of 305 subjects at 22 centers in the US. Subjects undergoing elective cardiovascular, neurologic (spinal) or general surgical procedures were randomized (stratified by surgical specialty) when there was oozing or bleeding of mild intensity that could not be controlled by other surgical techniques and the surgeon determined that a topical hemostatic agent was necessary. Bovine thrombin and EVITHROM® were applied with SURGIFOAM* Absorbable Gelatin Sponge, USP.
Treatment with EVITHROM® was as successful as treatment with bovine thrombin in achieving the primary efficacy endpoint: hemostasis within 10 minutes of product application and secondary efficacy endpoints: hemostasis within 6 and 3 minutes of product application.
| Time Interval | Treatment Group: # Successes/N (%) | Ratio Human/Bovine | 95% CI for Ratio Human/Bovine*,† |
|
|---|---|---|---|---|
| EVITHROM®
N=153 | Bovine thrombin N=152 |
|||
| 10 minutes | 149/153 (97.4) | 148/152 (97.4) | 1.00 | 0.96, 1.05 |
| 6 minutes | 145/153 (94.8) | 141/152 (92.8) | 1.02 | 0.96, 1.09 |
| 3 minutes | 112/153 (73.2) | 110/152 (72.4) | 1.01 | 0.88, 1.16 |
| Surgical Specialty | Treatment Group: # Successes/N (%) | Ratio Human/Bovine | 95% CI for Ratio Human/Bovine*,† |
|
|---|---|---|---|---|
| EVITHROM® | Bovine thrombin | |||
| Cardiovascular | 44/47 (93.6) | 38/46 (82.6) | 1.13 | 0.97, 1.36 |
| Neurosurgical (Spine) | 60/61 (98.4) | 59/60 (98.3) | 1.00 | 0.93, 1.08 |
| General Surgery | 41/45 (91.1) | 44/46 (95.7) | 0.95 | 0.82, 1.08 |
| Overall | 145/153 (94.8) | 141/152 (92.8) | 1.02 | 0.96, 1.09 |
At the 6 minute and 10 minute time points, >90% of subjects from all surgeries in both study groups had achieved hemostasis. The following results were documented for the 3 minute time point as stratified by surgery and study treatment: (1) cardiovascular surgery- human thrombin: 61.7%; bovine thrombin: 63.0%, (2) spinal surgery- human thrombin: 83.6%; bovine thrombin: 80.0%, (3) general surgery- human thrombin: 71.1%; bovine thrombin: 71.7%. for an overall ratio of proportions of 1.01.
16 HOW SUPPLIED/STORAGE AND HANDLING
EVITHROM® is supplied in the following single-use packages, each containing 800-1200 units/ml Thrombin, Topical (Human):
- Vial containing 2 ml, 5 ml or 20 ml frozen solution
Storage and handling
- The vials must be stored in an upright position.
- Store frozen vials at -18°C or colder for up to 2 years.
- Unopened vials can be stored at 2°C to 8°C for up to 30 days.
- EVITHROM® has been shown to be stable for up to 24 hours at room temperature.
- Do not use after the expiration date stated on the box or after 30 days if stored at 2°C to 8°C after thawing.
- Do not re-freeze EVITHROM® once it has been thawed.
- Do not refrigerate EVITHROM® once at room temperature.
- Discard unused product after 24 hours at room temperature.
- Discard if the packaging of EVITHROM® is damaged.
17 PATIENT COUNSELING INFORMATION
Some viruses such as hepatitis A virus and parvovirus B19 are particularly difficult to remove or inactivate. Parvovirus B19 most seriously affects pregnant women or immunocompromised individuals. Symptoms of parvovirus B19 infection include: fever, drowsiness, chills and runny nose followed about two weeks later by a rash and joint pain. Evidence of hepatitis A may include several days to weeks of poor appetite, fatigue and low-grade fever followed by nausea, vomiting and abdominal pain. Dark urine and a yellowed complexion are also common symptoms. Consult your physician if such symptoms appear.
If absorbed systemically EVITHROM® could potentially cause blood clotting disorders. Consult your physician for any new or unusual symptoms.
Distributed by:
Ethicon, Inc.
P.O. Box 151, Somerville, NJ 08876-0151 USA
Manufactured by:
Omrix Biopharmaceuticals Ltd.
MDA blood bank,
Sheba Hospital, Ramat-Gan
POB 888, Kiryat Ono 55000, ISRAEL
U.S. License No. 1603
© Ethicon, Inc. 2012
Art. No. 80TZ00M3D6
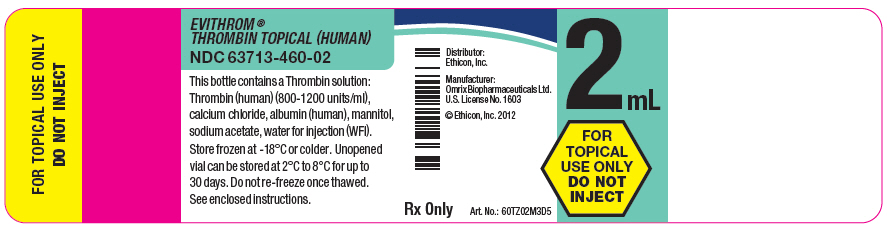
PRINCIPAL DISPLAY PANEL - 2 mL Vial Label
EVITHROM®
THROMBIN TOPICAL (HUMAN)
NDC 63713-460-02
This bottle contains a Thrombin solution:
Thrombin (human) (800-1200 units/ml),
calcium chloride, albumin (human), mannitol,
sodium acetate, water for injection (WFI).
Store frozen at -18°C or colder. Unopened
vial can be stored at 2°C to 8°C for up to
30 days. Do not re-freeze once thawed.
See enclosed instructions.
| EVITHROM
human thrombin liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Ethicon Inc (002144145) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Omrix Biopharmaceuticals Ltd. PFI | 514577949 | api manufacture(63713-460) , analysis(63713-460) , label(63713-460) , pack(63713-460) | |