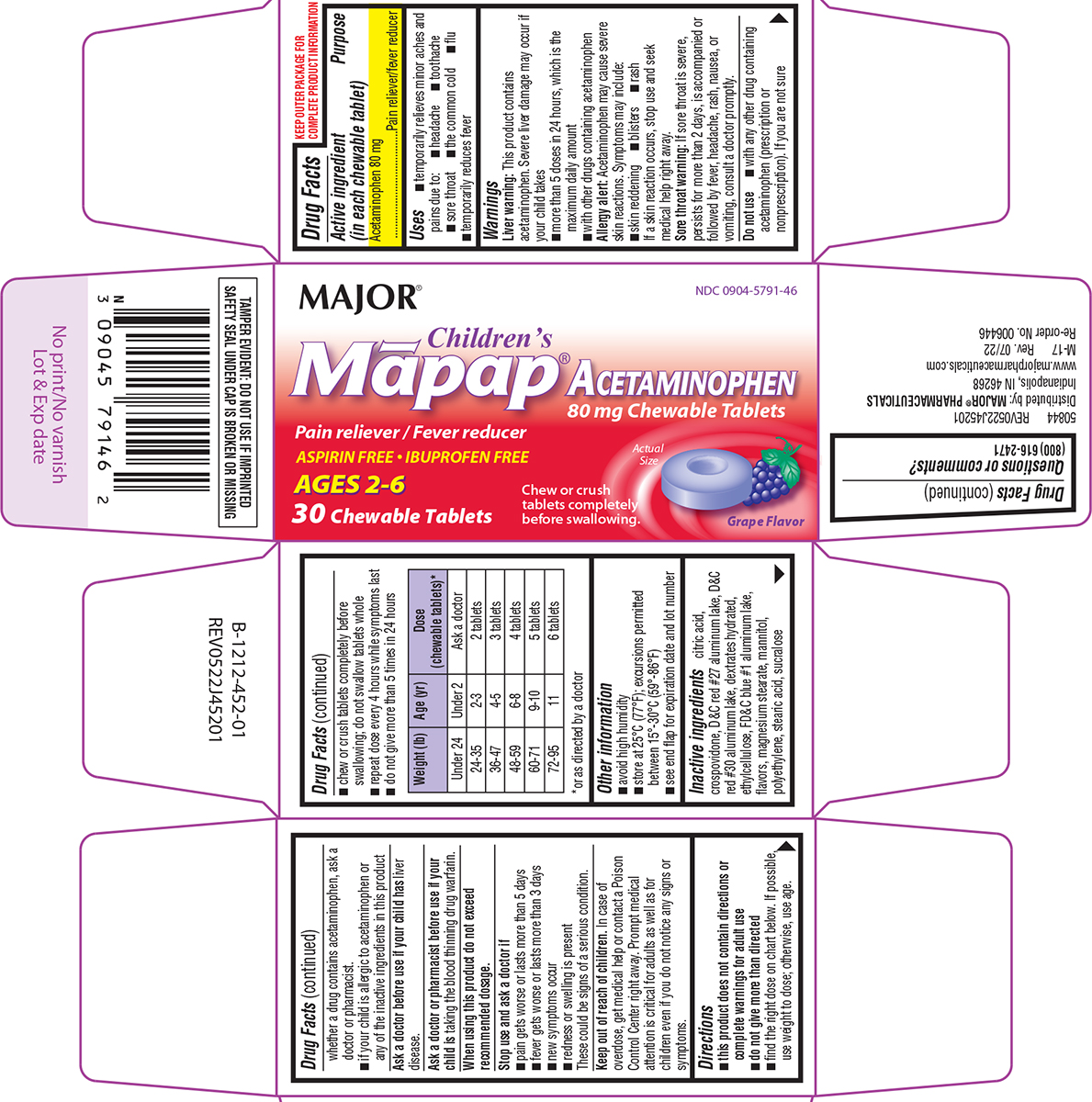
Label: CHILDRENS MAPAP- acetaminophen tablet, chewable
- NDC Code(s): 0904-5791-46
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated September 21, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
-
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
Weight (lb) Age (yr) Dose
(chewable tablets)*Under 24 Under 2 Ask a doctor 24-35 2-3 2 tablets 36-47 4-5 3 tablets 48-59 6-8 4 tablets 60-71 9-10 5 tablets 72-95 11 6 tablets *or as directed by a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
MAJOR®
NDC 0904-5791-46
Children's
Māpap®
ACETAMINOPHEN
80 mg Chewable Tablets
Pain reliever / Fever reducerASPIRIN FREE • IBUPROFEN FREE
AGES 2-6
30 Chewable Tablets
Actual Size
Chew or crush
tablets completely
before swallowing.
Grape Flavor
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING50844 REV0522J45201
Distributed by: MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268
www.majorpharmaceuticals.com
Major 44-452
-
INGREDIENTS AND APPEARANCE
CHILDRENS MAPAP
acetaminophen tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-5791 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 80 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color purple Score no score Shape ROUND Size 13mm Flavor GRAPE Imprint Code 44;452 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-5791-46 1 in 1 CARTON 12/06/2004 1 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 12/06/2004 Labeler - Major Pharmaceuticals (191427277) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(0904-5791) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(0904-5791) , pack(0904-5791) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(0904-5791) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(0904-5791)