Label: SALONPAS-HOT CAPSICUM- capsaicin patch
- NDC Code(s): 46581-700-01, 46581-700-03
- Packager: Hisamitsu Pharmaceutical Co., Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 17, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
Do not use
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
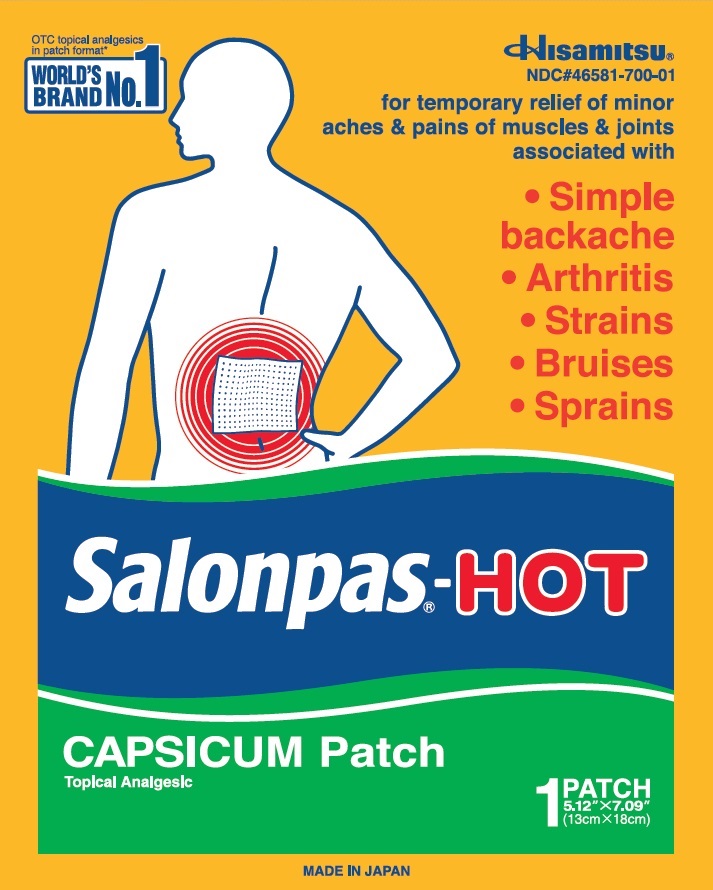
Principal Display Panel
OTC topical analgesics in patch format*
WORLD'S BRAND NO.1Hisamitsu
NDC#46581-700-01
for temporary relief of minor aches and pains of muscles and joints associated with
Simple backache
Arthritis
Strains
Bruises
Sprains
Salonpas-HOTCAPSICUM Patch
Topical Analgesic
1 PATCH
5.12" X 7.09" (13cm X 18cm)
MADE IN JAPAN
-
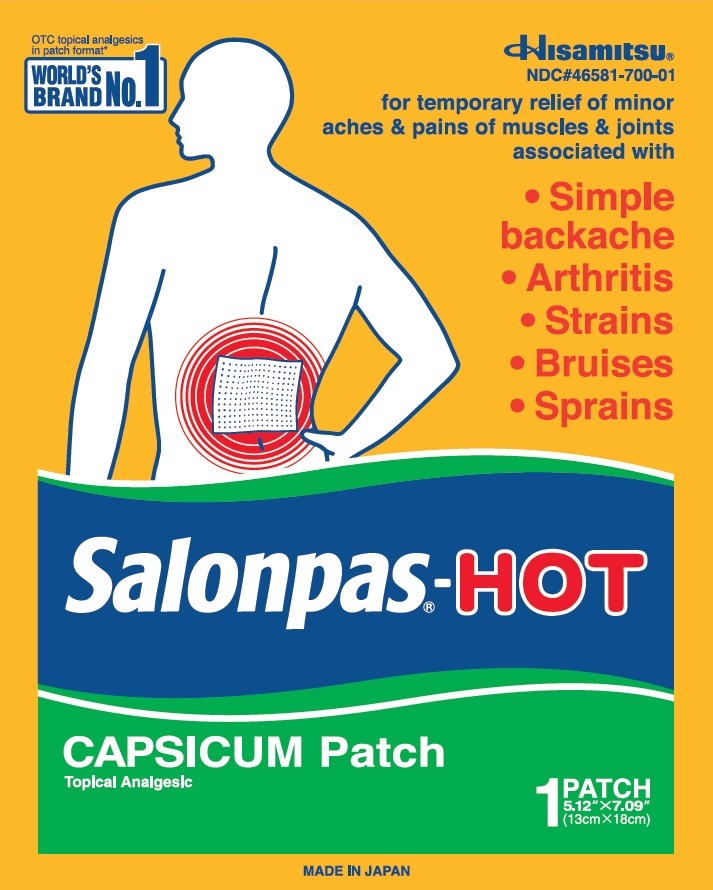
Principal Display Panel
3 LARGE FOLDED PATCHES
5.12" X 7.09"(13 cm X 18 cm)
OTC topical analgesics in patch category*
WORLD'S BRAND NO.1
Hisamitsu
NDC#46581-700-03
for temporary relief of minor aches and pains of muscles and joints associated with
Simple backache
Arthritis
Strains
Bruises
Sprains
Salonpas-HOT
CAPSICUM Patch
Topical Analgesic
3 FOLDED PATCHES
5.12" X 7.09" (13cm X 18cm)
MADE IN JAPAN
-
INGREDIENTS AND APPEARANCE
SALONPAS-HOT CAPSICUM
capsaicin patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46581-700 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSICUM (UNII: 00UK7646FG) (CAPSICUM - UNII:00UK7646FG) CAPSAICIN 0.025 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) NATURAL LATEX RUBBER (UNII: 2LQ0UUW8IN) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46581-700-01 1 in 1 POUCH 11/26/1998 1 4 g in 1 PATCH; Type 0: Not a Combination Product 2 NDC:46581-700-03 3 in 1 POUCH 12/01/2018 2 4 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/26/1998 Labeler - Hisamitsu Pharmaceutical Co., Inc. (690539713)