Label: PAYLEAN 45- ractopamine hydrochloride granule
- NDC Code(s): 58198-0610-9
- Packager: Elanco US Inc.
- Category: OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG LABEL
Drug Label Information
Updated July 11, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
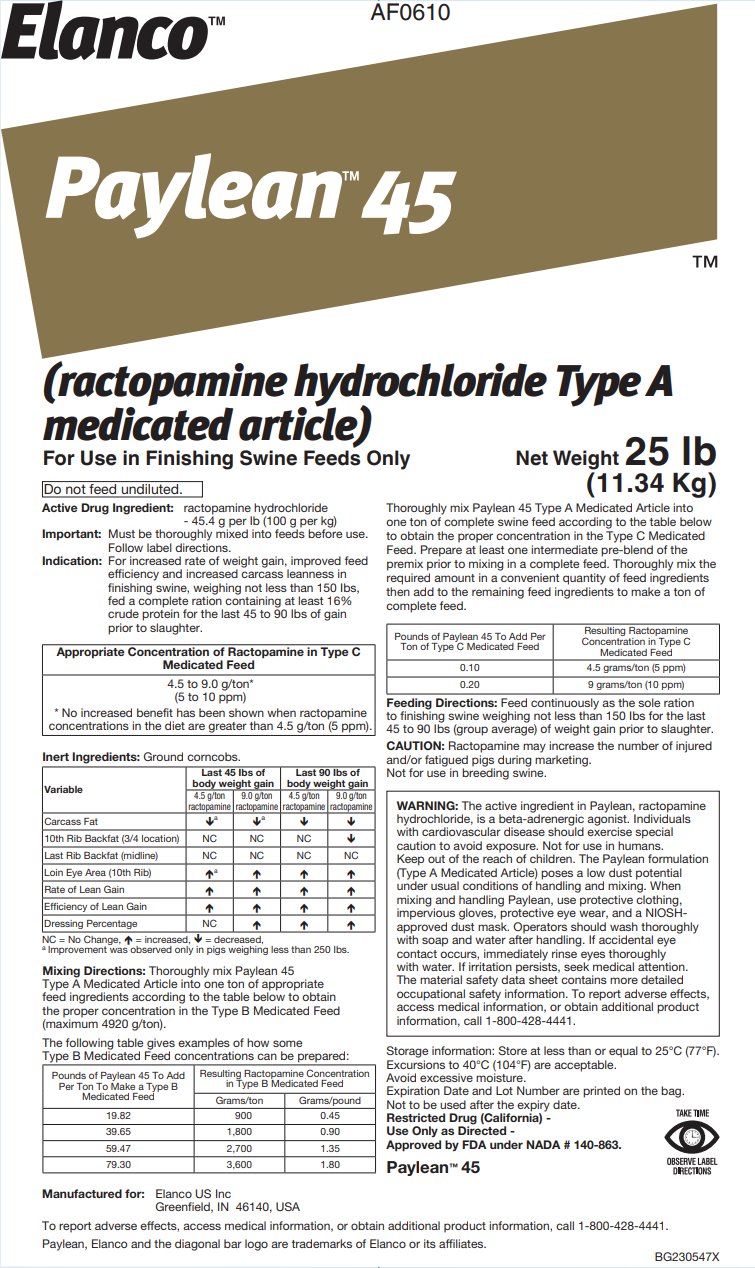
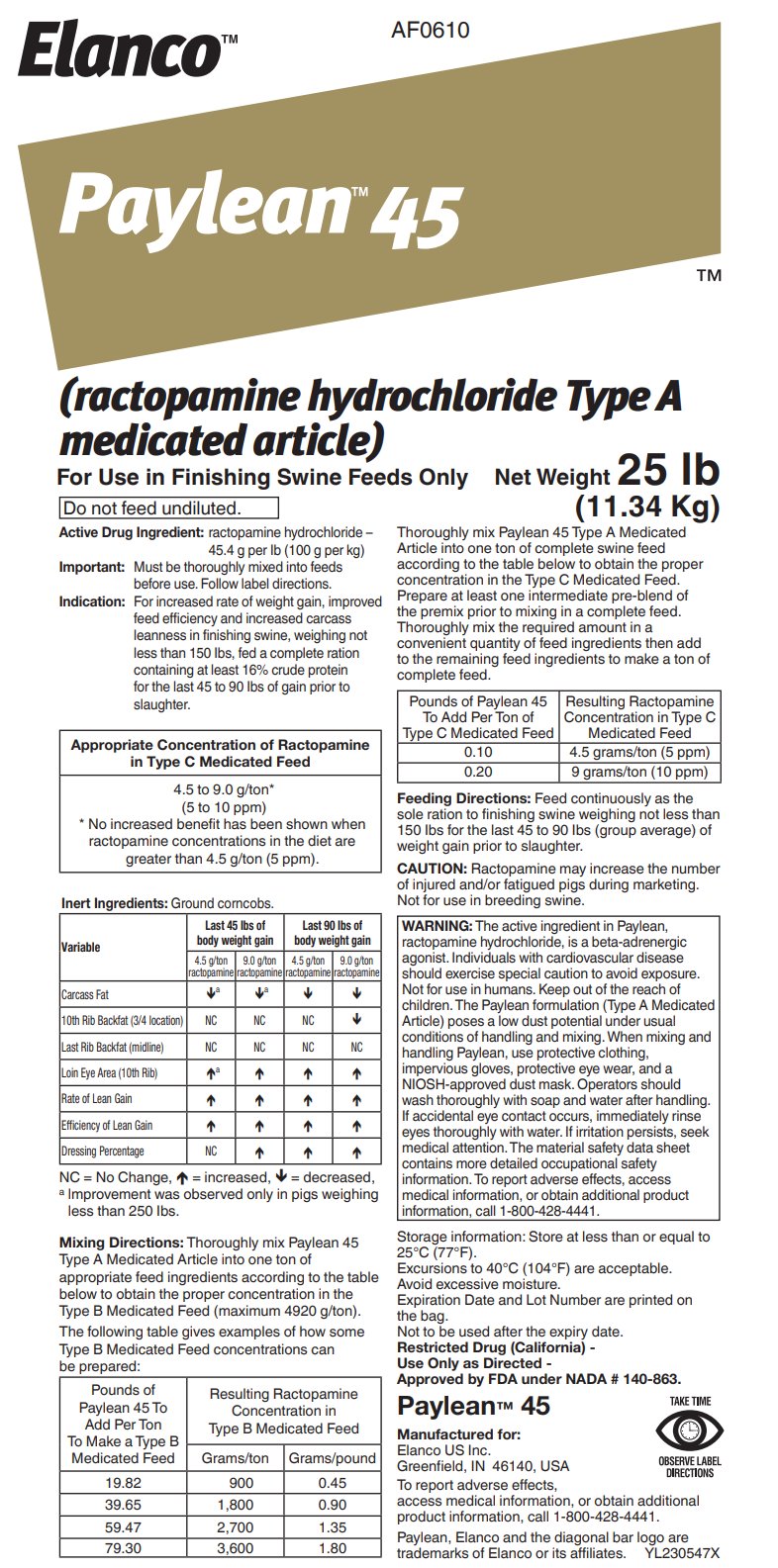
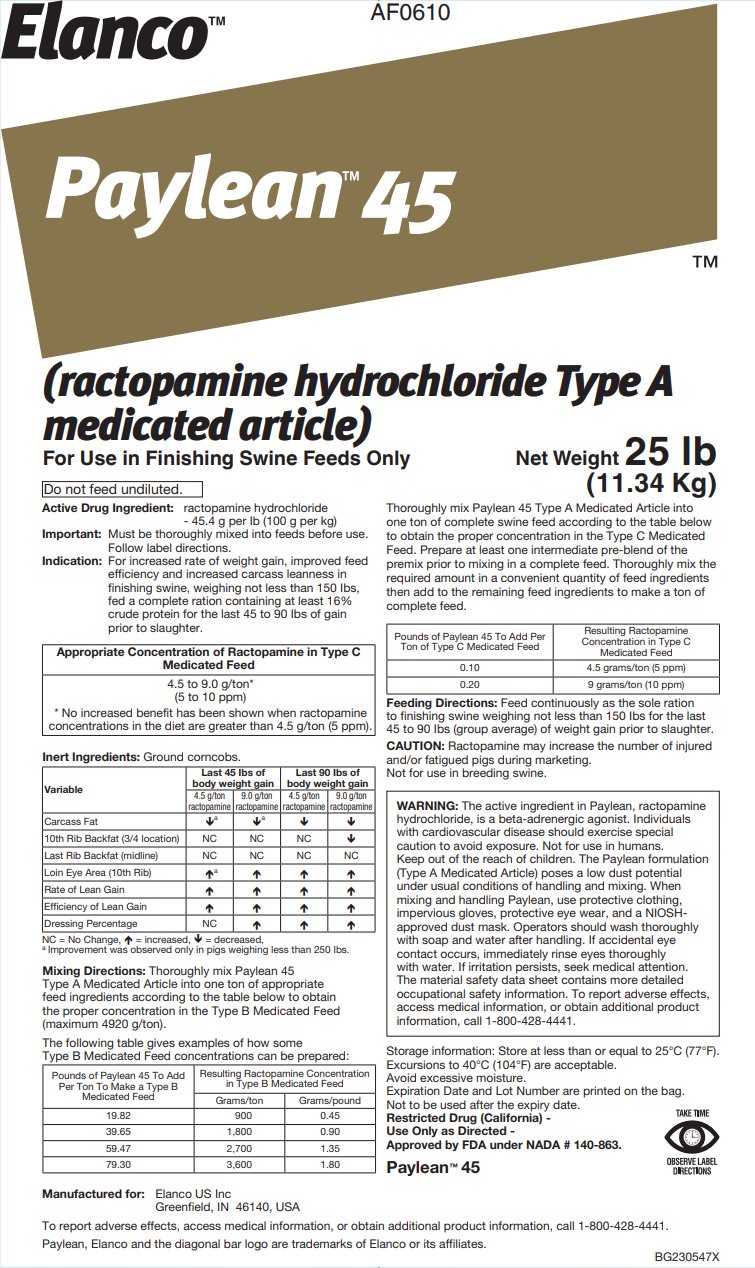
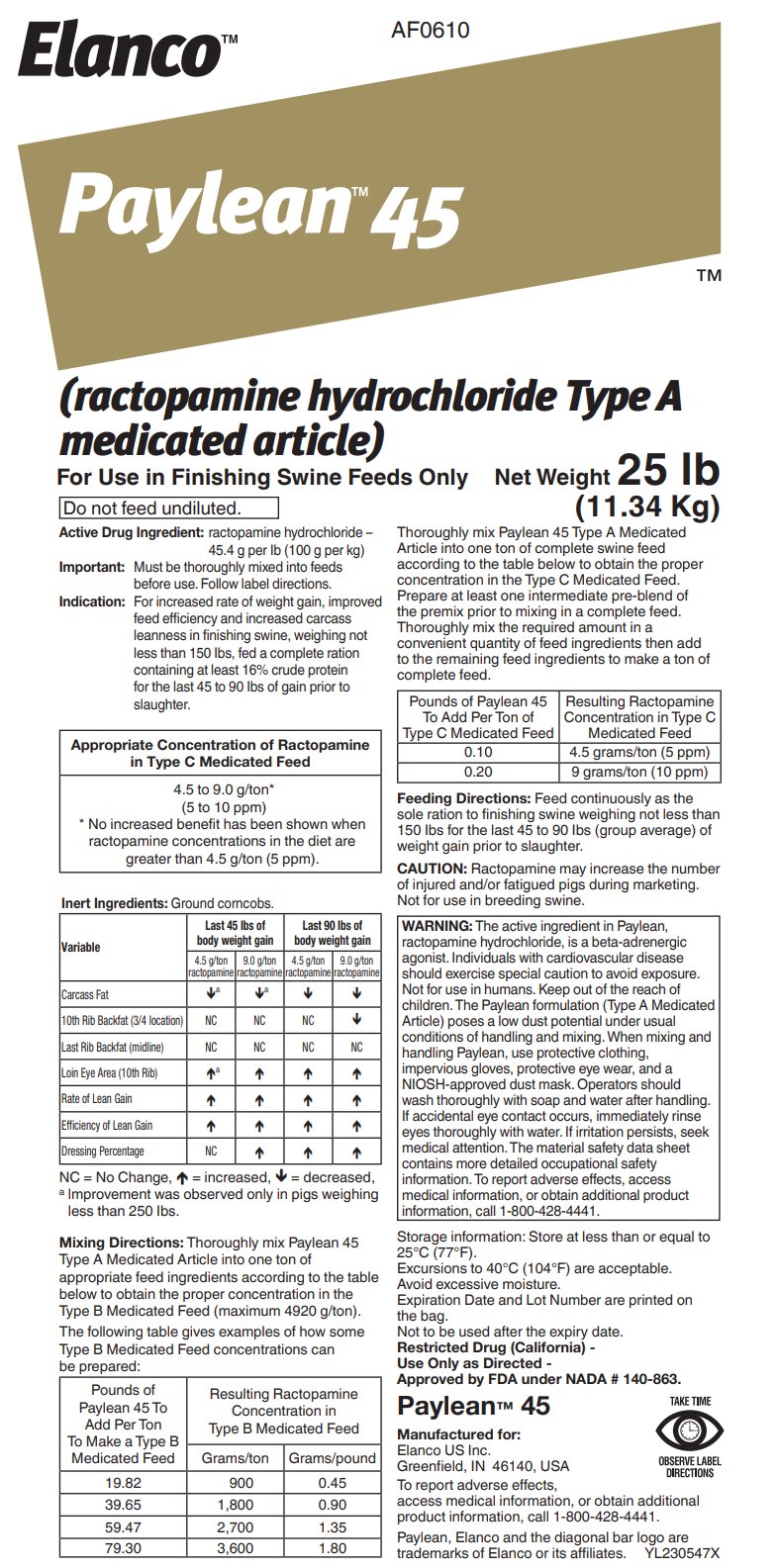
- Active Drug Ingredient:
-
Indication:
For increased rate of weight gain, improved feed efficiency and increased carcass leanness in finishing swine, weighing not less than 150 lbs, fed a complete ration containing at least 16% crude protein for the last 45 to 90 lbs of gain prior to slaughter.
Appropriate Concentration of Ractopamine in Type C
Medicated Feed4.5 to 9.0 g/ton*
(5 to 10 ppm)
* No increased benefit has been shown when ractopamine concentrations in the diet are greater than 4.5 g/ton (5 ppm).Inert Ingredients: Ground corncobs.
NC = No Change, ↑ = increased, ↓ = decreased, a Improvement was observed only in pigs weighing less than 250 Ibs. Variable
Last 45 lbs of body weight gain
Last 90 lbs of body weight gain
4.5 g/ton ractopamine
9.0 g/ton ractopamine
4.5 g/ton ractopamine
9.0 g/ton ractopamine
Carcass Fat
↓a
↓a
↓
↓
10th Rib Backfat (3/4 location)
NC
NC
NC
↓
Last Rib Backfat (midline)
NC
NC
NC
NC
Loin Eye Area (10th Rib)
↑a
↑
↑
↑
Rate of Lean Gain
↑
↑
↑
↑
Efficiency of Lean Gain
↑
↑
↑
↑
Dressing Percentage
NC
↑
↑
↑
Mixing Directions: Thoroughly mix Paylean 45 Type A Medicated Article into one ton of appropriate feed ingredients according to the table below to obtain the proper concentration in the Type B Medicated Feed (maximum 4920 g/ton).
The following table gives examples of how some Type B Medicated Feed concentrations can be prepared:
Pounds of Paylean 45 To Add Per Ton To Make a Type B Medicated Feed
Resulting Ractopamine Concentration in Type B Medicated Feed
Grams/ton
Grams/pound
19.82
900
0.45
39.65
1,800
0.90
59.47
2,700
1.35
79.30
3,600
1.80
Thoroughly mix Paylean 45 Type A Medicated Article into one ton of complete swine feed according to the table below to obtain the proper concentration in the Type C Medicated Feed. Prepare at least one intermediate pre-blend of the premix prior to mixing in a complete feed. Thoroughly mix the required amount in a convenient quantity of feed ingredients then add to the remaining feed ingredients to make a ton of complete feed.
Pounds of Paylean 45 To Add Per Ton of Type C Medicated Feed
Resulting Ractopamine
Concentration in Type C
Medicated Feed0.10
4.5 grams/ton (5 ppm)
0.20
9 grams/ton (10 ppm)
Feeding Directions: Feed continuously as the sole ration to finishing swine weighing not less than 150 lbs for the last 45 to 90 lbs (group average) of weight gain prior to slaughter.
CAUTION: Ractopamine may increase the number of injured and/or fatigued pigs during marketing. Not for use in breeding swine.
-
BOXED WARNING
(What is this?)
WARNING: The active ingredient in Paylean, ractopamine hydrochloride, is a beta-adrenergic agonist. Individuals with cardiovascular disease should exercise special caution to avoid exposure. Not for use in humans. Keep out of the reach of children. The Paylean formulation (Type A Medicated Article) poses a low dust potential under usual conditions of handling and mixing. When mixing and handling Paylean, use protective clothing, impervious gloves, protective eye wear, and a NIOSH-approved dust mask. Operators should wash thoroughly with soap and water after handling. If accidental eye contact occurs, immediately rinse eyes thoroughly with water. If irritation persists, seek medical attention. The material safety data sheet contains more detailed occupational safety information. To report adverse effects, access medical information, or obtain additional product information, call 1-800-428-4441.
-
STORAGE AND HANDLING
Storage information: Store at less than or equal to 25°C (77°F).
Excursions to 40°C (104°F) are acceptable.
Avoid excessive moisture.
Expiration Date and Lot Number are printed on the bag.
Not to be used after the expiry date.
Restricted Drug (California)
- Use Only as Directed -
Approved by FDA under NADA # 140-863.
Paylean™ 45
TAKE TIME
OBSERVE LABEL
DIRECTIONSManufactured for:
Elanco US Inc
Greenfield, IN 46140, USATo report adverse effects, access medical information, or obtain additional product information, call 1-800-428-4441.
Paylean, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
BG230547X
- Principal Display Panel - Paylean 45 Bag Label
-
INGREDIENTS AND APPEARANCE
PAYLEAN 45
ractopamine hydrochloride granuleProduct Information Product Type OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC:58198-0610 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ractopamine hydrochloride (UNII: 309G9J93TP) (ractopamine - UNII:57370OZ3P1) ractopamine hydrochloride 45.5 g in 0.45 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58198-0610-9 11.34 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140863 06/06/2008 Labeler - Elanco US Inc. (966985624) Establishment Name Address ID/FEI Business Operations Evonik Corporation 130890994 API MANUFACTURE Establishment Name Address ID/FEI Business Operations TriRx Speke Limited 228138655 MANUFACTURE, PACK, LABEL