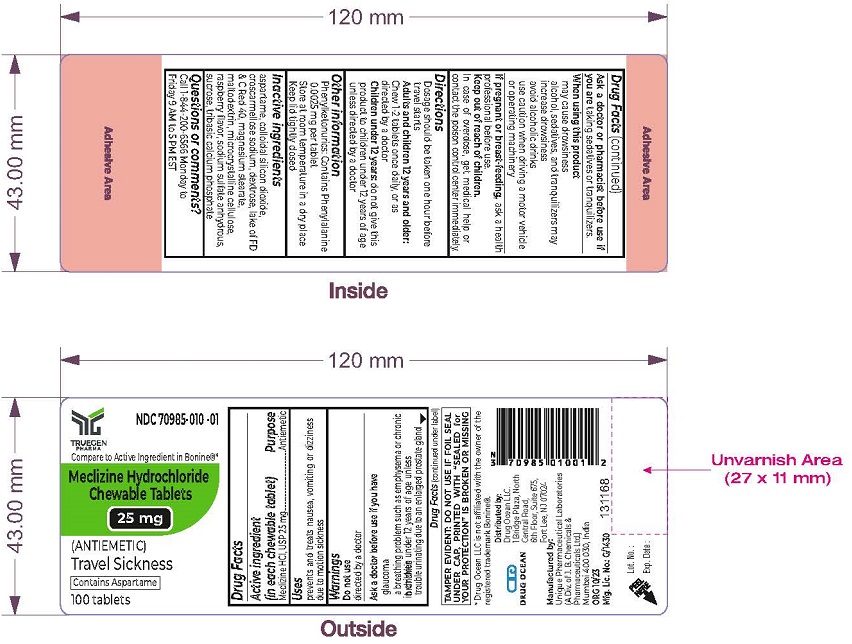
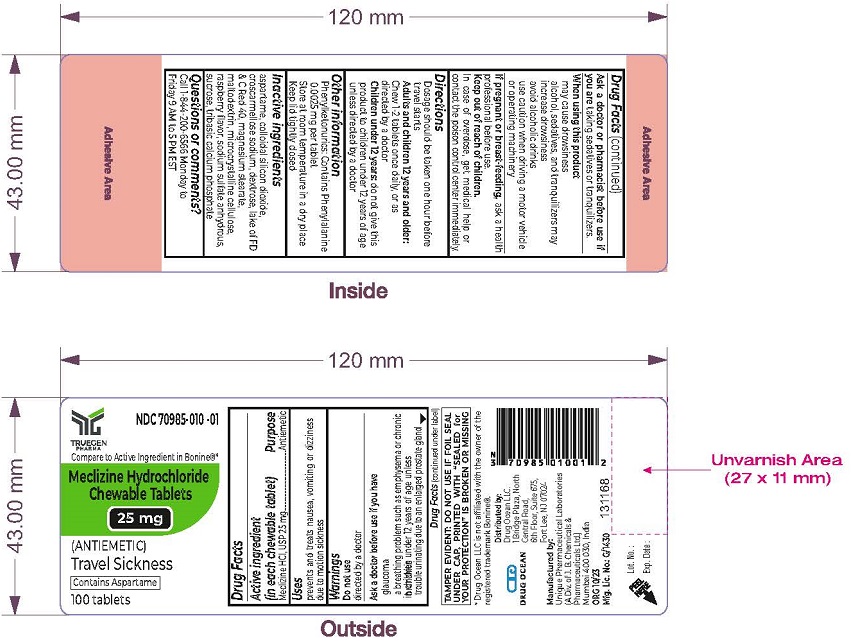
Label: MECLIZINE HCL- meclizine hydrochloride chewable tablet, chewable
- NDC Code(s): 70985-010-01
- Packager: Drug Ocean LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 23, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (in each chewable tablet)
- Purpose
- Uses
- Warnings
- ASK DOCTOR/PHARMACIST
- When using this product
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions or Comments?
-
SPL UNCLASSIFIED SECTION
TAMPER EVIDENT: DO NOT USE IF FOIL SEAL UNDER CAP, PRINTED WITH “SEALED for YOUR PROTECTION” IS BROKEN OR MISSING
Distributed by:

Drug Ocean LLC,
1 Bridge Plaza, North Central Road6th Floor, Suite 675
Fort Lee, NJ 07024
Manufactured by:
Unique Pharmaceutical Laboratories
(A Div. of J. B. Chemicals & Pharmaceuticals Ltd.)
Mumbai 400 030, IndiaORG 12/23
- PRINCIPAL DISPLAY PANEL - 25 mg Chewable Tablet Label
-
INGREDIENTS AND APPEARANCE
MECLIZINE HCL
meclizine hydrochloride chewable tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70985-010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) ASPARTAME (UNII: Z0H242BBR1) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DEXTROSE (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) RASPBERRY (UNII: 4N14V5R27W) SODIUM SULFATE ANHYDROUS (UNII: 36KCS0R750) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color pink (Pink to light pink) Score 2 pieces Shape ROUND Size 8mm Flavor RASPBERRY Imprint Code M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70985-010-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/28/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 02/28/2022 Labeler - Drug Ocean LLC (080381835) Registrant - Unique Pharmaceutical Laboratories (917165052) Establishment Name Address ID/FEI Business Operations Unique Pharmaceutical Laboratories 650434645 manufacture(70985-010)