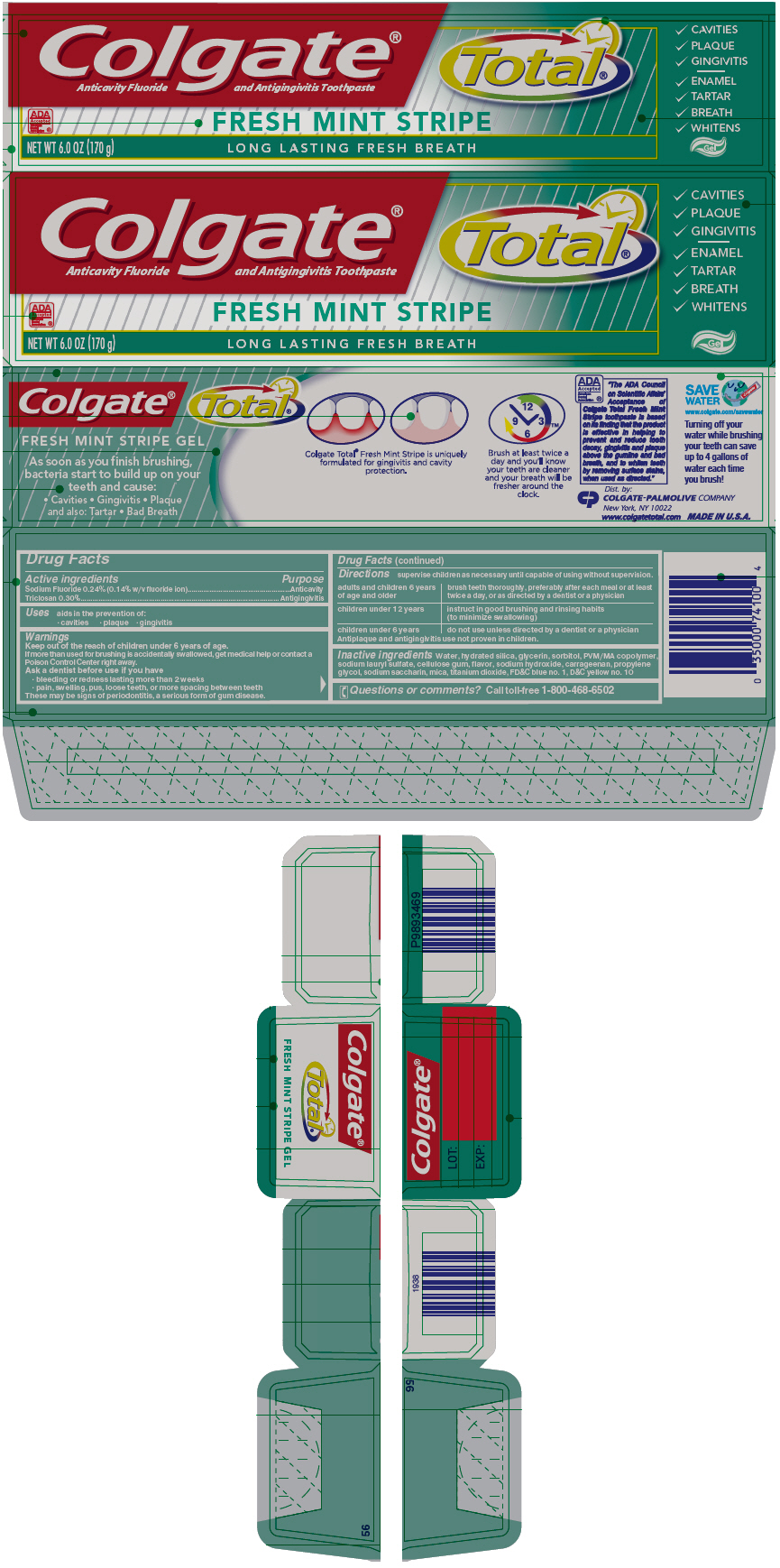
COLGATE TOTAL FRESH MINT STRIPE- sodium fluoride and triclosan paste, dentifrice
Colgate-Palmolive Company
----------
Colgate® Total® Fresh Mint Stripe Gel
Warnings
Directions
supervise children as necessary until capable of using without supervision.
| adults and children 6 years of age and older | brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or a physician |
| children under 12 years | instruct in good brushing and rinsing habits (to minimize swallowing) |
| children under 6 years | do not use unless directed by a dentist or a physician |
Antiplaque and antigingivitis use not proven in children.
| COLGATE TOTAL FRESH MINT STRIPE
sodium fluoride and triclosan paste, dentifrice |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Colgate-Palmolive Company (001344381) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Colgate-Palmolive | 785047999 | MANUFACTURE(35000-161) | |
Revised: 12/2019
Document Id: 5a8e437e-600f-4794-a5e6-bc15b63d7061
Set id: eeae522f-645c-4a16-8514-37907da323df
Version: 10
Effective Time: 20191209
Colgate-Palmolive Company