Label: SPECTRAMAST DC- ceftiofur hydrochloride suspension
- NDC Code(s): 54771-5278-1, 54771-5278-2
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated May 4, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
CAUTION
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian. Federal Law prohibits extra-label use of this drug in dry dairy cattle for disease prevention purposes; at unapproved doses, frequencies, durations, or routes of administration; and in unapproved major food producing species/production classes.
-
DESCRIPTION
Ceftiofur hydrochloride is a cephalosporin antibiotic.
Chemical Structure of Ceftiofur Hydrochloride 
U-64279A Chemical Name of Ceftiofur Hydrochloride
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7 - [[2-(2-amino-4-thiazolyl) - 2 -(methoxyimino)acetyl]amino]-3-[[(2-furanyl-carbonyl)thio]methyl]-8-oxo, hydrochloride.
Ceftiofur Hydrochloride Sterile Suspension is an oil based sterile suspension.
Each 10 mL PLASTET® Disposable Syringe Contains:
Ceftiofur Equivalents (as the hydrochloride salt) 500 mg Microcrystalline Wax 700 mg Oleoyl Polyoxylglyceride 500 mg Cottonseed Oil q.s. -
INDICATIONS FOR USE
SPECTRAMAST® DC Ceftiofur Hydrochloride Sterile Suspension is indicated for the treatment of subclinical mastitis in dairy cattle at the time of dry off associated with Staphylococcus aureus, Streptococcus dysgalactiae, and Streptococcus uberis.
SPECTRAMAST® DC Ceftiofur Hydrochloride Sterile Suspension has been proven effective against Staphylococcus aureus, Streptococcus dysgalactiae, and Streptococcus uberis.
-
DOSAGE
Infuse one (1) syringe into each affected quarter at the time of dry off.
DIRECTIONS FOR USING THE PLASTET® DISPOSABLE SYRINGE
The syringe is designed to provide the choice of either insertion of the full cannula as has traditionally been practiced, or insertion of no more than 1/8 inch of the cannula, as reported by Eberhart, R.J., et. al. 1987. Current Concepts of Bovine Mastitis, 3rd Edition, National Mastitis Council, Arlington, VA.
a. Full insertion: Remove the red end cap by pulling straight up as shown. Gently insert the full cannula into the teat canal; carefully infuse the product.
b. Partial insertion: Remove the red end cap by pulling straight up as shown. Gently insert the exposed white tip into the teat canal; carefully infuse the product.
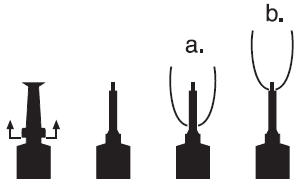
-
ADMINISTRATION
Treatment:
Wash teats thoroughly with warm water containing a suitable dairy antiseptic. Dry teats thoroughly. Milk out udder completely. Using an alcohol pad provided, wipe off the end of the affected teat using a separate pad for each teat. Choose the desired insertion length (full or partial) and insert tip into teat canal; push plunger to dispense entire contents, massage the quarter to distribute the suspension into the milk cistern.
- CONTRAINDICATIONS
- SPL UNCLASSIFIED SECTION
-
WARNINGS
Penicillins and cephalosporins can cause allergic reactions in sensitized individuals. Topical exposures to such antimicrobials, including ceftiofur, may elicit mild to severe allergic reactions in some individuals. Repeated or prolonged exposure may lead to sensitization. Avoid direct contact of the product with the skin, eyes, mouth and clothing. Sensitization of the skin may be avoided by wearing protective gloves.
Persons with a known hypersensitivity to penicillin or cephalosporins should avoid exposure to this product.
In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. If allergic reaction occurs (e.g., skin rash, hives, difficult breathing), seek medical attention.
The Safety Data Sheet contains more detailed occupational safety information. For a copy of the Safety Data Sheet or to report adverse reactions, call Zoetis Inc. at 1-888-963-8471. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or at www.fda.gov/reportanimalae.
-
RESIDUE WARNINGS
- Milk taken from cows completing a 30-day dry cow period may be used for food with no milk discard due to ceftiofur residues.
- Following label use, no pre-slaughter withdrawal period is required for neonatal calves born from treated cows regardless of colostrum consumption.
- Following intramammary infusion, a 16-day pre-slaughter withdrawal period is required for treated cows.
- Use of this product in a manner other than indicated under DOSAGE might result in violative residues.
-
CLINICAL MICROBIOLOGY
Ceftiofur is a broad-spectrum cephalosporin antibiotic that exerts its effect by inhibiting bacterial cell wall synthesis. Like other ß-lactam antimicrobial agents, the cephalosporins inhibit cell wall synthesis by interfering with the enzymes essential for peptidoglycan synthesis. This effect results in lysis of the bacterial cell and accounts for the bactericidal nature of these agents. Ceftiofur has demonstrated in vitro activity against clinical isolates and isolates from diagnostic laboratories. The results of susceptibility testing of these isolates against ceftiofur are presented in Tables 1 and 2. Appropriate reference strains were also susceptibility tested and their minimum inhibitory concentration (MIC) values and zone of inhibition with a 30 μg disk are presented in Table 4.
Table 1. Ceftiofur MIC values for isolates from a multi-site clinical field study evaluating subclinical mastitis in dry dairy cows in the U.S. during 2000 Organism No. MIC90*
(μg/mL)MIC range
(μg/mL)- *
- The MIC for 90% of the isolates.
Staphylococcus aureus 300 1.0 ≤0.06 to 2.0 Streptococcus dysgalactiae 55 ≤0.06 ≤0.06 to >64.0 Streptococcus uberis 58 1.0 ≤0.06 to 4.0 * The MIC for 90% of the isolates.Table 2. Ceftiofur MIC values* for mastitis pathogens from diagnostic laboratories in the U.S. and Canada Organism No. Date isolated MIC90†
(μg/mL)MIC range
(μg/mL)Staphylococcus
aureus135 1991–1992 1.0 0.13 to 2.0 10 1993 1.0 0.25 to 1.0 107 1995 1.0 0.25 to 2.0 61 2000 1.0 ≤0.06 to 2.0 Coagulase (-)
staphylococci139 2000–2001 1.0 ≤0.06 to 2.0 Streptococcus
dysgalactiae15 1991–1992 1.0 ≤0.06 to 2.0 15 1993 ≤0.0039 No range‡ 152 1997–1999 0.25 0.25 to 4.0 64 2000 ≤0.06 ≤0.06 to 0.5 Streptococcus
uberis22 1991–1992 0.5 ≤0.06 to 4.0 15 1993 0.03 ≤0.0039 to 0.06 133 1997–1999 0.5 0.5 to 8.0 20 2000 1.0 <0.06 to 2.0 Escherichia coli 39 1991–1992 1.0 0.25 to 1.0 40 1993 0.5 0.13 to 1.0 52 2000 0.5 ≤0.06 to 1.0 * The above in vitro data are available, but their clinical significance is unknown.
** The MIC for 90% of the isolates.
† No range, all isolates yielded the same value.Based on pharmacokinetic, milk residue and clinical effectiveness studies in dairy cattle following intramammary infusion of ceftiofur and the MIC and disk (30 μg) diffusion data from mastitis pathogens, the following breakpoints are recommended by the National Committee for Clinical Laboratory Standards [now the Clinical and Laboratories Standards Institute (CLSI)] (Table 3).
Table 3. Current recommended interpretive criteria established by CLSI for ceftiofur for Bovine Mastitis Bovine Mastitis
OrganismsDisk
ContentZone Diameter
(mm)MIC breakpoint
(μg/mL)S I R S I R S – Susceptible I – Intermediate R – Resistant Staphylococcus
aureusStreptococcus
dysgalactiaeStreptococcus
uberis
30 μg
≥21
18–20
≤17
≤2.0
4.0
≥8.0Streptococcus
agalactiaeEscherichia coli Standardized procedures require the use of laboratory control organisms for both standardized diffusion techniques and standardized dilution techniques. The 30 μg ceftiofur sodium disk should give the following zone diameters and the ceftiofur sodium standard reference powder (or disk) should provide the following MIC values for the reference strain. The ceftiofur sodium disks or standard reference powder is appropriate for ceftiofur hydrochloride (Table 4).
S – Susceptible
I – Intermediate
R – ResistantTable 4. Acceptable quality control ranges for ceftiofur against CLSI recommended American Type Culture Collection (ATCC) reference strains Organism (ATCC No.) Zone Diameter*
(mm)MIC range
(μg/mL)- *
- All testing performed using a 30 μg disk.
Escherichia coli (25922) 26 to 31 0.25 to 1.0 Staphylococcus aureus (29213) --- 0.25 to 1.0 Staphylococcus aureus (25923) 27 to 31 --- Pseudomonas aeruginosa (27853) 14 to 18 16.0 to 64.0 *All testing performed using a 30 μg disk.
-
EFFECTIVENESS
The effectiveness of a single intramammary (IMM) infusion of ceftiofur hydrochloride for the treatment of subclinical mastitis present at the time of dry off was demonstrated in a randomized block design study. Nineteen veterinary investigators enrolled cows in 21 herds and from these 21 herds, 431 cows and 1708 quarters met enrollment criteria in the study and calved within a 45 to 60 day period following enrollment. The enrollment criteria were whole udder somatic cell counts greater than 400,000 cells/mL or a linear somatic cell count score greater than or equal to 5. Milk microbiologic samples were obtained prior to treatment and at Days 3 and 5 post-calving. There were 5 treatment groups including a negative control group. There were 43 cows in the negative control group and 51 cows in the 500 mg ceftiofur group that had a positive pre-treatment milk culture that were evaluated for treatment success. The primary decision variable was the microbiologic (therapeutic) cure in which bacteria isolated pre-treatment were absent from both posttreatment samples.
In another study in eleven study herds, 446 cows with a somatic cell count (SCC) greater than or equal to 400,000 cells/mL or a linear score greater than or equal to 5 were enrolled. Cows with a dry period of at least 45 days were blocked by lactation (1st + 2nd or ≥3rd). A single quarter milk sample was aseptically obtained from all four quarters for bacterial culture prior to treatment and on Days 3 and 5 post-calving. There were 4 treatment groups including a negative control. There were 84 cows in the negative control and 73 in the 500 mg ceftiofur group that had a positive pre-treatment milk culture that were evaluated for treatment success. The primary decision variable was the microbiologic (therapeutic) cure in which bacteria isolated pre-treatment were absent from both post-treatment samples.
Ceftiofur was found to be effective against Staphylococcus aureus, Streptococcus dysgalactiae, and Streptococcus uberis, when compared to negative controls. This intramammary ceftiofur formulation was well tolerated. No adverse formulation related events were noted during the entire study. A large multi-location field dose confirmation study and a pilot study demonstrated that 500 mg of ceftiofur infused once per quarter at the time of dry off was effective for the treatment of subclinical mastitis in dairy cattle at the time of dry off.
-
ANIMAL SAFETY
An udder irritation study was conducted in 22 healthy lactating dairy cows to assess udder irritation following a single intramammary infusion of a sterile oil-based suspension containing 500 mg of ceftiofur into all four quarters followed by milk-out 12 hours later. Throughout the 10 day post-treatment observation period there was a clinically insignificant rise in SCC to mean levels <200,000 cells/mL from the pre-infusion level of <69,000 cells/mL. No clinical signs of udder irritation (swelling, pain, or redness), changes in rectal temperature, or changes in milk production were noted in this study. Clinical observations were made during a GLP residue depletion study of 36 cows following a single intramammary infusion of a sterile oil based suspension containing 500 mg of ceftiofur into all four quarters at the end of lactation. No report of udder irritation or adverse reaction was noted in the daily visual observations over the 14 days immediately following treatment. Collectively, these studies demonstrate that the intramammary infusion of an oil-based sterile suspension containing 500 mg of ceftiofur once into all four quarters at the end of lactation is clinically safe and non-irritating to the udder of non-lactating dairy cows.
-
MILK AND TISSUE RESIDUE DEPLETION
A metabolism study in cattle using radiolabeled ceftiofur provided the data to establish tolerances for ceftiofur-related residues (as desfuroylceftiofur) in tissue and milk. These tolerances of ceftiofur residues are 0.1 ppm in milk, 0.4 ppm in kidney, 2.0 ppm in liver, and 1.0 ppm in muscle.
Pivotal residue decline studies were conducted to assess the depletion of ceftiofur-related residues, measured as desfuroylceftiofur using the official analytical method, in tissues of treated cows, in milk from treated cows, and in tissues of calves born to treated cows. In these studies, non-mastitic cows received 500 mg of ceftiofur per quarter into all four quarters once at dry off. The milk residue depletion study demonstrated that milk produced at calving may be used for human consumption with no discard period when the treatment to calving interval is 30 days or more. The tissue depletion study measured residues in the tissues of treated cows and in the tissues of neonatal calves born to treated cows. In neonatal calves born to treated cows, tissue residues were less than the codified tolerances for kidney, liver and muscle. These data support a zero day pre-slaughter withdrawal period for calves born to treated cows when the treatment to calving interval is 30 days or more, regardless of colostrum consumption. The tissue residue depletion data support a 16-day pre-slaughter withdrawal period following intramammary infusion for treated cows.
- STORAGE CONDITIONS
-
HOW SUPPLIED
SPECTRAMAST® DC Sterile Suspension is available in cartons containing 1 unbroken package of 12–10 mL PLASTET® Disposable Syringes with 12 individually wrapped 70% isopropyl alcohol pads and in pails containing 12 unbroken packages of 12-10 mL PLASTET Disposable Syringes with 144 individually wrapped 70% isopropyl alcohol pads.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 12 Syringe Carton
- PRINCIPAL DISPLAY PANEL - 144 Syringe Pail Label
-
INGREDIENTS AND APPEARANCE
SPECTRAMAST DC
ceftiofur hydrochloride suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-5278 Route of Administration INTRAMAMMARY Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFTIOFUR HYDROCHLORIDE (UNII: 6822A07436) (CEFTIOFUR - UNII:83JL932I1C) CEFTIOFUR 500 mg in 10 mL Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-5278-1 12 in 1 CARTON 1 10 mL in 1 SYRINGE 2 NDC:54771-5278-2 144 in 1 PAIL 2 10 mL in 1 SYRINGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141239 03/15/2005 Labeler - Zoetis Inc. (828851555)




