DIABETIC SILTUSSIN DAS-NA- guaifenesin liquid
Lannett Company, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
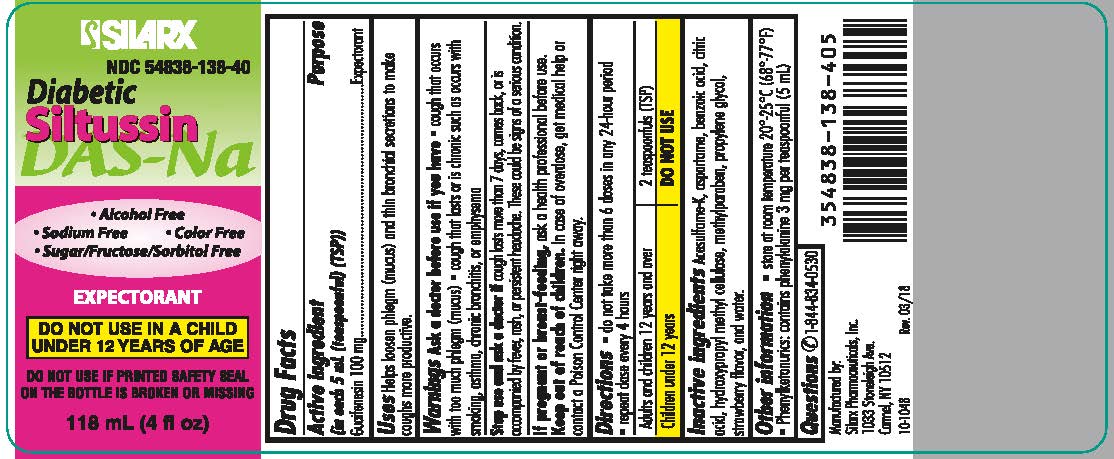
Diabetic Siltussin DAS-Na
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
Directions
- do not take more than 6 doses in any 24-hour period repeat dose every 4 hours
| Adults and children 12 years and over | 2 teaspoonfuls (TSP) |
| Children under 12 years | DO NOT USE |
Other information
store at room temperature 20°-25°C (68°-77°F)
Phenylketonurics: contains phenylalanine 3 mg per teaspoonful (5 mL)
Inactive Ingredients
Acesulfame-K, aspartame, benzoic acid, citric acid, hydroxypropyl methyl cellulose, methylparaben, propylene glycol, strawberry flavor, and water.
| DIABETIC SILTUSSIN DAS-NA
guaifenesin liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Lannett Company, Inc. (002277481) |