SALONPAS PAIN RELIEVING HOT- capsaicin, menthol patch
Hisamitsu Pharmaceutical Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
For temporary relief of minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only
Do not use
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- do not use otherwise than as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
- discontinue use at least 1 hour before a bath or shower
- do not use immediately after a bath or shower
Directions
Adults and children 12 years of age and over:
- clean and dry affected area
- remove film from patch and apply to the skin (see illustration)
- apply to affected area not more than 3 to 4 times daily
- remove patch from the skin after at most 8 hours' application
Children under 12 years of age: consult a doctor
Inactive ingredients
aluminum silicate, edetate disodium, gelatin, glycerin, magnesium aluminometasilicate, oleyl alcohol, polyacrylic acid, polyethylene glycol, polyvinyl alcohol, sodium polyacrylate, sorbitan monooleate, tartaric acid, titanium dioxide, water
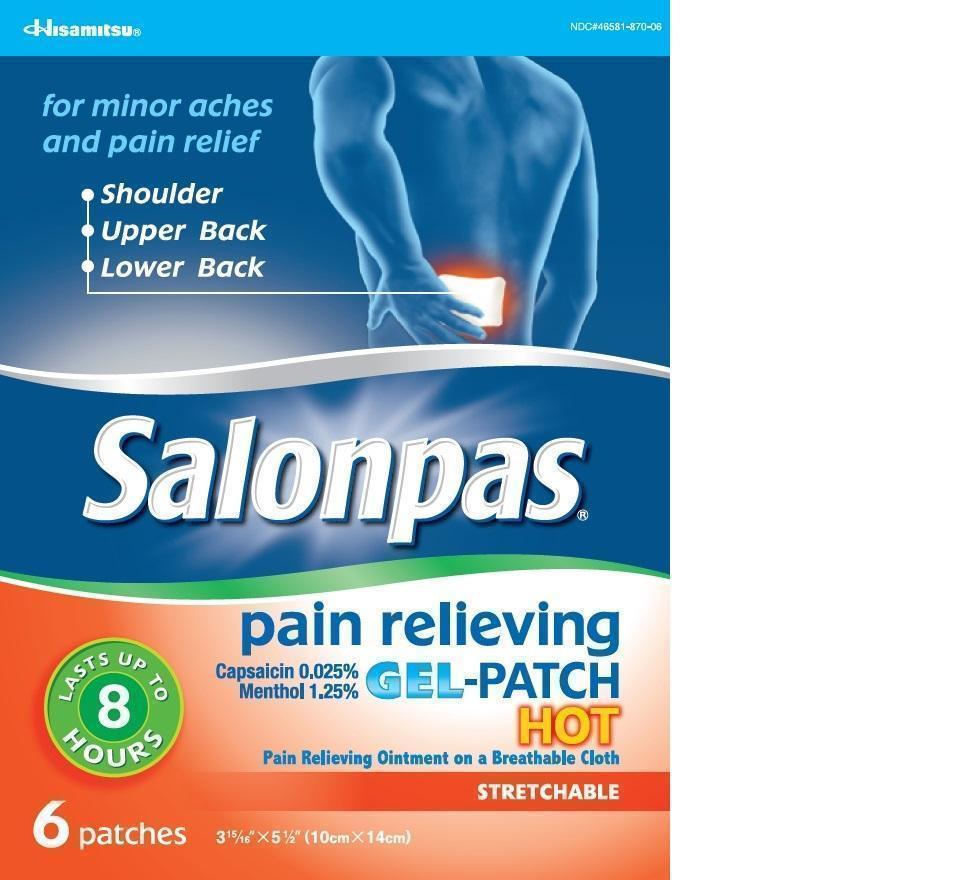
Principal Display Panel
Hisamitsu
NDC#46581-870-06
for minor aches and pain relief
- Shoulder
- Upper Back
- Lower Back
Salonpas pain relieving GEL-PATCH HOT
Capsaicin 0.025%
Menthol 1.25%
LASTS UP TO 8 HOURS
Pain Relieving Ointment on a Breathable Cloth
STRETCHABLE
6 patches 3 15/16 X 5 1/2 (10cm X 14cm)
Principal Display Panel

NDC#46581-870-99
for minor aches and pain relief
- Shoulder
- Upper Back
- Lower Back
1 Pull apart.
2 Place on affected area.
3 Slide the both films.
4 Gently apply the patch.
Salonpas pain relieving GEL-PATCH HOT
Capsaicin 0.025%
Menthol 1.25%
LASTS UP TO 8 HOURS
Pain Relieving Ointment on a Breathable Cloth
STRETCHABLE
1 patch 3 15/16" X 5 1/2" (10cm X 14cm)
Hisamitsu
Manufactured by
Hisamitsu Pharmaceutical Co., Inc.
JAPAN SAGA TOSU
MADE IN JAPAN
Distributed by
Hisamitsu America, Inc.
Florham Park, NJ 07932
FREE SAMPLE NOT FOR RE-SALE
| SALONPAS
PAIN RELIEVING HOT
capsaicin, menthol patch |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Hisamitsu Pharmaceutical Co., Inc. (690539713) |