CORRECTOR 2- hydroquinone cream
Vivier Pharma Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Corrector 2 Hydroquinone Cream
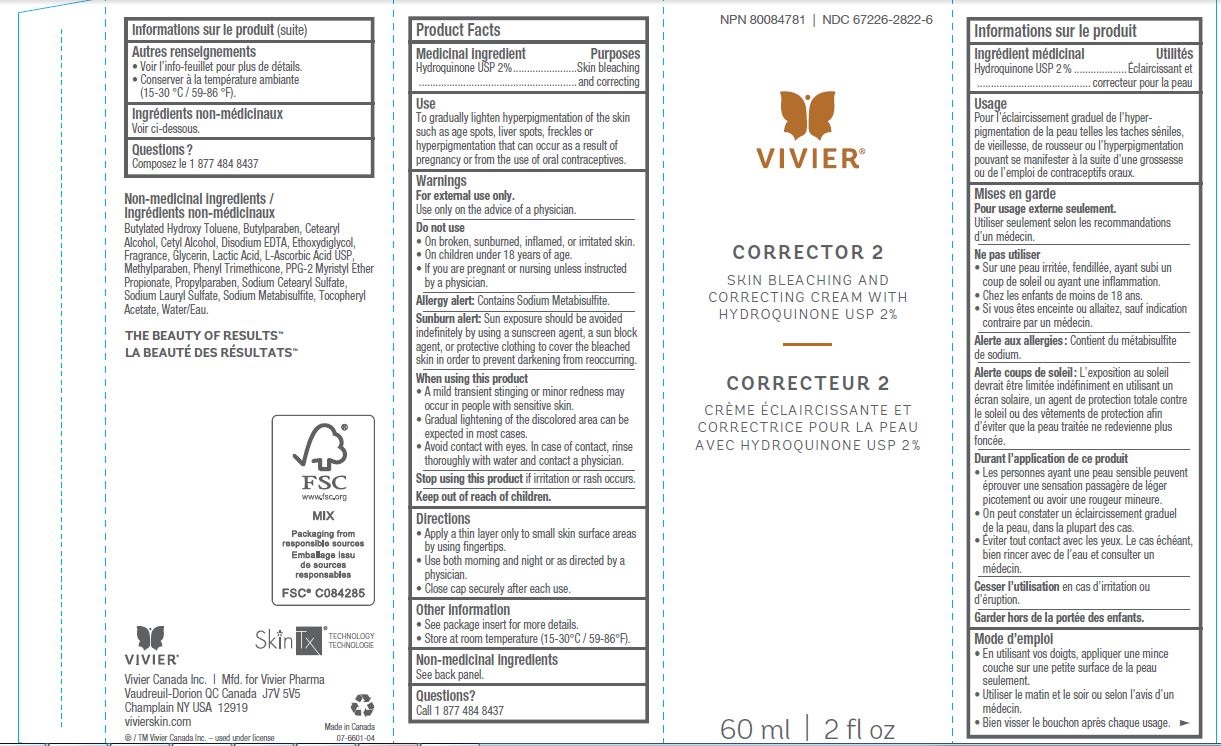
USE
- To gradually lighten hyperpigmentation of the skin such as age spots, liver spots, freckles or hyperpigmentation that can occur as a result of pregnancy or from the use of oral contraceptives.
WARNINGS
For external use only.Use only on the advice of a physician.
Do not use
- On broken or irritated skin.
- On children under 12 years of age.
- If you are pregnant or nursing unless instructed by a physician.
Sunburn alert:
Limit sun exposure during treatment by using protective clothing or a sunscreen protective agent to avoid re-darkening of the skin.
DIRECTIONS
- Use fingertips to apply a thin layer to affected areas.
- Use both morning and night or as directed by a physician.
- Close cap securely after each use.
NON-MEDICINAL INGREDIENTS
Butylated Hydroxy Toluene, Butylparaben, Cetearyl Alcohol, Cetyl Alcohol, Disodium EDTA, Ethoxydiglycol, Fragrance, Glycerin, Lactic Acid, L-Ascorbic Acid USP, Methylparaben, Phenyl Trimethicone, PPG-2 Myristyl Ether Propionate, Propylparaben, Sodium Cetearyl Sulfate, Sodium Lauryl Sulfate, Sodium Metabisulfite, Tocopheryl Acetate, Water/Eau.
| CORRECTOR 2
hydroquinone cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Vivier Pharma Inc. (250996550) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Entreprises ImportFAB Inc. | 248586117 | manufacture(67226-2822) | |