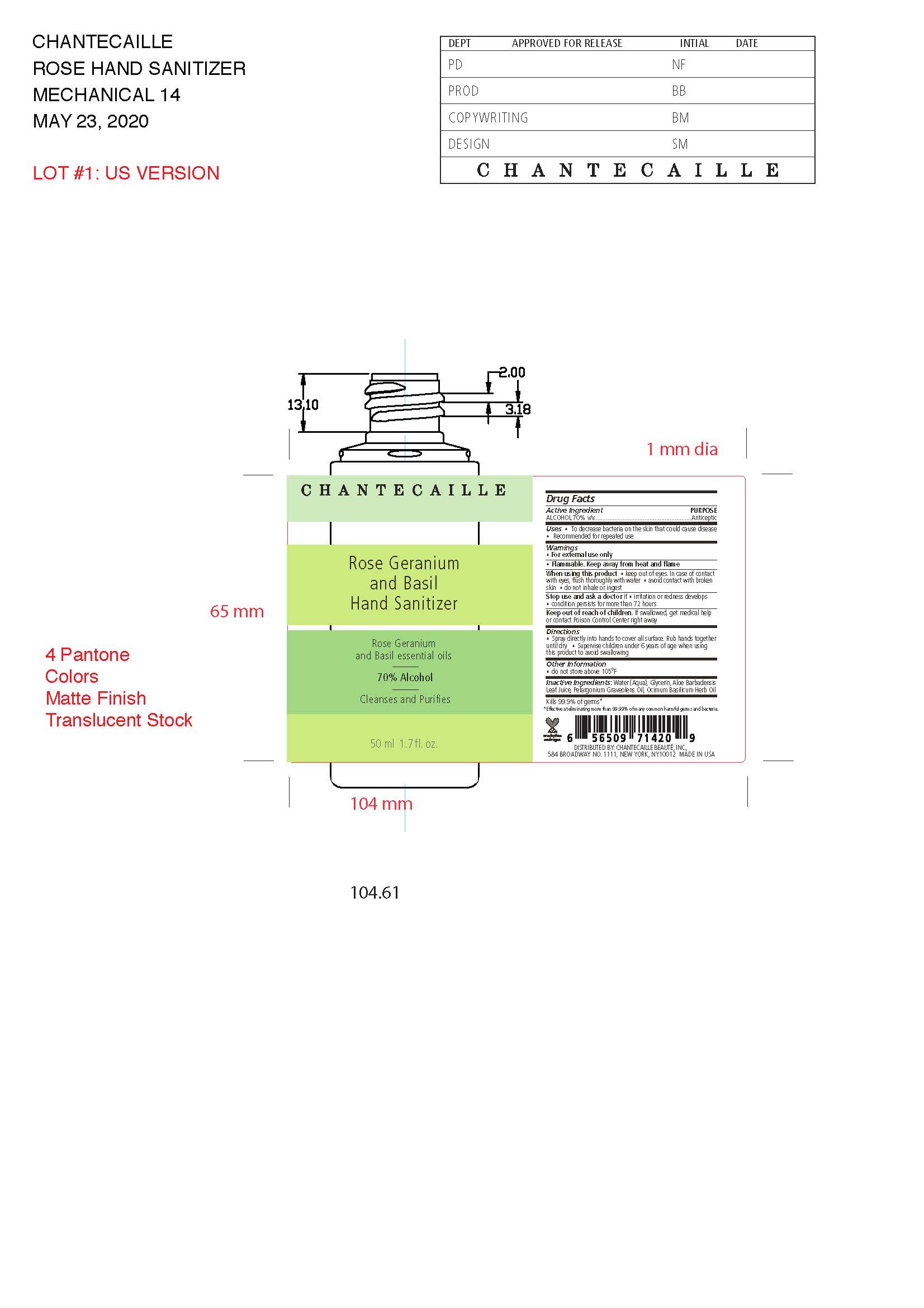
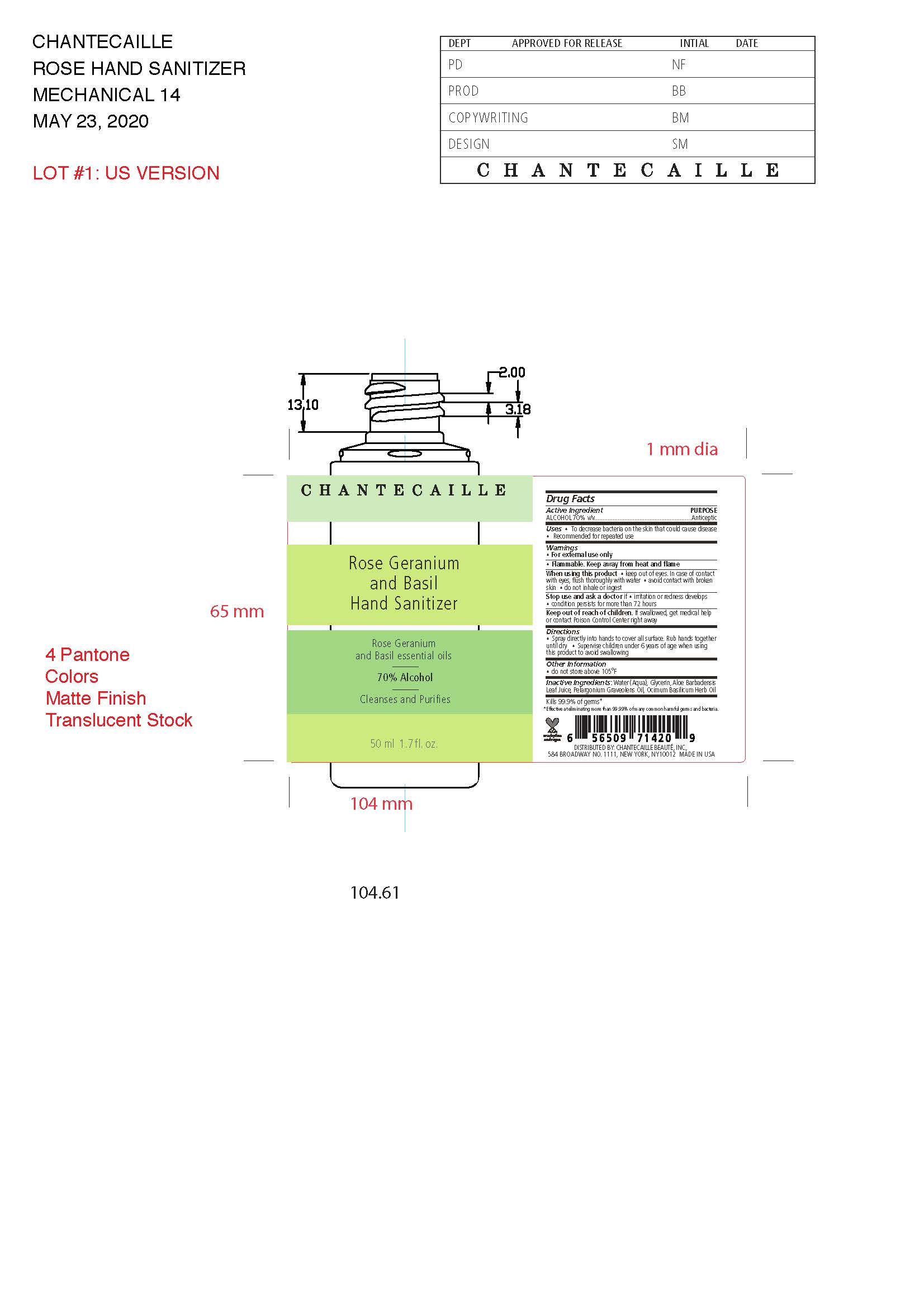
Label: CHANTECAILLE ROSE GERANIUM AND BASIL HAND SANITIZER solution
- NDC Code(s): 42893-100-50
- Packager: Chantecaille Beaute Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
-
WHEN USING
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Prinicpal Display Panel
-
INGREDIENTS AND APPEARANCE
CHANTECAILLE ROSE GERANIUM AND BASIL HAND SANITIZER
chantecaille rose geranium and basil hand sanitizer solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42893-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) OCIMUM BASILICUM WHOLE (UNII: P4815JL4O3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42893-100-50 1 in 1 CARTON 07/01/2020 05/17/2024 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/29/2020 05/17/2024 Labeler - Chantecaille Beaute Inc. (095270166)