BEVYXXA- betrixaban capsule, gelatin coated
Portola Pharmaceuticals LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BEVYXXA safely and effectively. See full prescribing information for BEVYXXA.
BEVYXXA® (betrixaban) capsules, for oral use Initial U.S. Approval: 2017 WARNING: SPINAL/EPIDURAL HEMATOMASee full prescribing information for complete boxed warning.Epidural or spinal hematomas may occur in patients treated with betrixaban who are receiving neuraxial anesthesia or undergoing spinal puncture. The risk of these events may be increased by the use of in-dwelling epidural catheters or the concomitant use of medical products affecting hemostasis. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. (5.2) INDICATIONS AND USAGEBEVYXXA is a factor Xa (FXa) inhibitor indicated for the prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness who are at risk for thromboembolic complications due to moderate or severe restricted mobility and other risk factors for VTE. (1) Limitations of Use Safety and efficacy of BEVYXXA have not been established in patients with prosthetic heart valves because this population has not been studied. (1) DOSAGE AND ADMINISTRATIONThe recommended dose of BEVYXXA is an initial single dose of 160 mg, followed by 80 mg once daily, taken at the same time each day with food. The recommended duration of treatment is 35 to 42 days. (2.1) DOSAGE FORMS AND STRENGTHSCapsules: 40 mg and 80 mg (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reaction (incidence > 5%) is bleeding. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Portola Pharmaceuticals at 1-866-777-5947 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 8/2020 |
FULL PRESCRIBING INFORMATION
WARNING: SPINAL/EPIDURAL HEMATOMA
Epidural or spinal hematomas may occur in patients treated with betrixaban who are receiving neuraxial anesthesia or undergoing spinal puncture. The risk of these events may be increased by the use of in-dwelling epidural catheters or the concomitant use of medical products affecting hemostasis. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures [see Warnings and Precautions (5.2)].
1 INDICATIONS AND USAGE
BEVYXXA is indicated for the prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness who are at risk for thromboembolic complications due to moderate or severe restricted mobility and other risk factors for VTE [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose of BEVYXXA is an initial single dose of 160 mg, followed by 80 mg once daily. Daily oral doses should be given at the same time of day with food.
The recommended duration of treatment is 35 to 42 days.
2.2 Use with P-gp Inhibitors
For patients receiving or starting concomitant P-gp inhibitors, the recommended dose of BEVYXXA is an initial single dose of 80 mg, followed by 40 mg once daily [see Warnings and Precautions (5.4), Drug Interactions (7.1), Clinical Pharmacology (12.3)]. The recommended duration of treatment is 35 to 42 days.
2.3 Patients with Severe Renal Impairment
For patients with severe renal impairment (CrCl ≥ 15 to < 30 mL/min computed by Cockcroft-Gault using actual body weight), the recommended dose of BEVYXXA is an initial single dose of 80 mg, followed by 40 mg once daily [see Warnings and Precautions (5.3), Use in Specific Populations (8.6), Clinical Pharmacology (12.3)]. The recommended duration of treatment is 35 to 42 days.
2.4 Patients with Hepatic Impairment
Avoid use of BEVYXXA in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3)]. No dose adjustment is required in patients with mild hepatic impairment.
3 DOSAGE FORMS AND STRENGTHS
Capsules:
- 80 mg (size 2) hard gelatin capsules have a light grey body with 80 printed in black and a blue cap with PTLA printed in white.
- 40 mg (size 4) hard gelatin capsules have a light grey body with 40 printed in black and a light blue cap with PTLA printed in white.
4 CONTRAINDICATIONS
BEVYXXA is contraindicated in patients with:
- Active pathological bleeding [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)]
- Severe hypersensitivity reaction to betrixaban [see Adverse Reactions (6.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Bleeding
BEVYXXA increases the risk of bleeding and can cause serious and potentially fatal bleeding. Promptly evaluate any signs or symptoms of blood loss [see Adverse Reactions (6.1)].
Concomitant use of drugs affecting hemostasis increases the risk of bleeding. These include aspirin and other antiplatelet agents, other anticoagulants, heparin, thrombolytic agents, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and nonsteroidal anti-inflammatory drugs (NSAIDs) [see Drug Interactions (7.3)].
Advise patients of signs and symptoms of blood loss and to report them immediately and seek emergency care. Promptly evaluate any signs or symptoms of blood loss, and consider the need for blood replacement. Discontinue BEVYXXA in patients with active pathological bleeding.
There is no established way to reverse the anticoagulant effect of BEVYXXA, which can be expected to persist for at least 72 hours after the last dose. It is unknown whether hemodialysis removes BEVYXXA. Protamine sulfate, vitamin K, and tranexamic acid are not expected to reverse the anticoagulant activity of BEVYXXA.
5.2 Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis.
Do not remove an epidural catheter earlier than 72 hours after the last administration of BEVYXXA. Do not administer the next BEVYXXA dose earlier than 5 hours after the removal of the catheter. If traumatic puncture occurs, delay the administration of BEVYXXA for 72 hours.
Monitor patients frequently for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, bowel or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention, consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
5.3 Use in Patients with Severe Renal Impairment
Patients with severe renal impairment (CrCl ≥ 15 to < 30 mL/min computed by Cockcroft-Gault using actual body weight) taking BEVYXXA may have an increased risk of bleeding events. Reduce dose of BEVYXXA, monitor patients closely, and promptly evaluate any signs or symptoms of blood loss in these patients [see Dosage and Administration (2.3), Warnings and Precautions (5.1), Adverse Reactions (6.1), Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
5.4 Use in Patients on Concomitant P-gp Inhibitors
Patients on concomitant P-gp inhibitors with BEVYXXA may have an increased risk of bleeding. Reduce dose of BEVYXXA in patients receiving or starting P-gp inhibitors. Monitor patients closely, and promptly evaluate any signs or symptoms of blood loss in these patients [see Dosage and Administration (2.2), Warnings and Precautions (5.1), Adverse Reactions (6.1), Drug Interactions (7.1), Clinical Pharmacology (12.3)].
Avoid use of BEVYXXA in patients with severe renal impairment receiving concomitant P-gp inhibitors [see Warnings and Precautions (5.3)].
5.5 Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome
Direct-acting oral anticoagulants (DOACs), including BEVYXXA, are not recommended for use in patients with triple-positive antiphospholipid syndrome (APS). For patients with APS (especially those who are triple positive [positive for lupus anticoagulant, anticardiolipin, and anti–beta 2- glycoprotein I antibodies]), treatment with DOACs has been associated with increased rates of recurrent thrombotic events compared with vitamin K antagonist therapy.
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk of Bleeding [see Warnings and Precautions (5.1, 5.3, 5.4)].
- Spinal/Epidural Anesthesia or Puncture [see Boxed Warning and Warnings and Precautions (5.2)].
- Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome [see Warnings and Precautions (5.5)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of BEVYXXA was evaluated in the Acute Medically Ill Prevention with Extended Duration Betrixaban (APEX) Study [see Clinical Studies (14)], including 3,716 patients treated with BEVYXXA for a median of 36 days compared to 3,716 patients treated with enoxaparin for a median of 9 days. Patients in both treatment groups were followed for safety, including bleeding events, for up to 77 days.
Patients randomized to the BEVYXXA arm received BEVYXXA 160 mg orally on Day 1, then 80 mg once daily for 35 to 42 days AND enoxaparin subcutaneous placebo once daily for 6 to 14 days. Patients randomized to the enoxaparin arm received enoxaparin 40 mg subcutaneously once daily for 6 to 14 days AND BEVYXXA placebo orally once daily for 35 to 42 days.
Patients with severe renal impairment (creatinine clearance ≥ 15 and < 30 mL/min) received reduced doses of study medications (BEVYXXA 80 mg loading dose, then 40 mg once daily or enoxaparin 20 mg once daily) along with corresponding placebo.
Patients taking a concomitant P-gp inhibitor received BEVYXXA 80 mg loading dose, then 40 mg once daily or enoxaparin 40 mg subcutaneously once daily for 6 to 14 days along with corresponding placebo.
Hemorrhage
The most common adverse reactions with BEVYXXA were related to bleeding (> 5%), with major bleeding occurring in less than 1% of patients (see Table 1).
Overall, 54% of patients receiving BEVYXXA experienced at least one adverse reaction versus 52% with enoxaparin. The frequency of patients reporting serious adverse reactions was similar between BEVYXXA (18%) and enoxaparin (17%). In the APEX trial, the most frequent reason for treatment discontinuation was bleeding, with an incidence rate of 2.4% for BEVYXXA versus 1.2% for enoxaparin.
The primary and secondary safety outcomes in APEX were bleeding-related events.
A summary of major and clinically relevant non-major (CRNM) bleeding events in the overall safety population is shown in Table 1. Most CRNM events (86%) were mild to moderate in severity, and the majority (62%) did not require medical intervention.
The incidence of fatal bleeding was the same in the BEVYXXA and enoxaparin treatment groups (1 in each group).
| Parameter | BEVYXXA (N=3,716) | Enoxaparin (N=3,716) | BEVYXXA vs. Enoxaparin RR (95% CI) |
|---|---|---|---|
|
|||
| Major Bleeding * | 25 (0.67) | 21 (0.57) | 1.19 (0.67, 2.12) p = 0.554 |
| Gastrointestinal (GI) | 19 (0.51) | 9 (0.24) | |
| Intracranial Hemorrhage | 2 (0.05) | 7 (0.19) | |
| Intraocular | 0 (0) | 1 (0.03) | |
| Fatal Bleeding | 1 (0.03) | 1 (0.03) | |
| CRNM † | 91 (2.45) | 38 (1.02) | 2.39 (1.64, 3.49) p < 0.001 |
A summary of major and CRNM bleeding events by dose is shown in Table 2 and Table 3.
| Parameter | BEVYXXA 80 mg (N=2,986) n (%) | Enoxaparin 40 mg (N=2,991) n (%) |
|---|---|---|
| Major | 15 (0.50) | 16 (0.53) |
| RR (95% CI) | 0.94 (0.47, 1.90) | |
| CRNM | 66 (2.21) | 33 (1.10) |
| RR (95% CI) | 2.00 (1.32, 3.03) | |
| Major or CRNM | 81 (2.71) | 49 (1.64) |
| RR (95% CI) | 1.66 (1.17, 2.35) | |
| Severe Renal Impairment | Concomitant Use of P-gp Inhibitor | |||
|---|---|---|---|---|
| Parameter | BEVYXXA 40 mg (N=150) n (%) | Enoxaparin 20 mg (N=125) n (%) | BEVYXXA 40 mg (N=542) n (%) | Enoxaparin 40 mg (N=527) n (%) |
| Major | 3 (2.00) | 1 (0.80) | 6 (1.11) | 4 (0.76) |
| RR (95% CI) | 2.5 (0.26, 23.74) | 1.46 (0.41, 5.14) | ||
| CRNM | 6 (4.00) | 2 (1.60) | 20 (3.69) | 3 (0.57) |
| RR (95% CI) | 2.5 (0.51, 12.17) | 6.5 (1.94, 21.68) | ||
| Major or CRNM | 9 (6.00) | 3 (2.40) | 26 (4.80) | 7 (1.33) |
| RR (95% CI) | 2.5 (0.69, 9.04) | 3.6 (1.58, 8.25) | ||
The most common adverse reactions occurring in ≥ 2% of patients are shown in Table 4.
| Adverse Reaction | BEVYXXA N=3,716 n (%) | Enoxaparin N=3,716 n (%) |
|---|---|---|
| Bleeding-related (all sources) | ||
| Epistaxis | 58 (2) | 24 (1) |
| Hematuria | 62 (2) | 28 (1) |
| Non-bleeding Adverse Reaction | ||
| Urinary Tract Infection | 123 (3) | 87 (2) |
| Constipation | 110 (3) | 102 (3) |
| Hypokalemia | 93 (3) | 84 (2) |
| Hypertension | 89 (2) | 80 (2) |
| Headache | 74 (2) | 59 (2) |
| Nausea | 67 (2) | 56 (2) |
| Diarrhea | 64 (2) | 61 (2) |
7 DRUG INTERACTIONS
7.1 Inhibitors of P-gp
Betrixaban is a substrate of P-gp, and concomitant use of P-gp inhibitors (e.g., amiodarone, azithromycin, verapamil, ketoconazole, or clarithromycin) results in an increased exposure of betrixaban [see Clinical Pharmacology (12.3)].
Reduce the dose of BEVYXXA for patients receiving or starting concomitant P-gp inhibitors [see Dosage and Administration (2.2), Warnings and Precautions (5.4), Clinical Pharmacology (12.3)].
7.2 Inducers of P-gp
Co-administration of BEVYXXA with P-gp inducers (e.g., carbamazepine, phenytoin, rifampin, or St. John's wort) may result in a decrease in systemic exposure of betrixaban and its pharmacodynamic effects. Therefore, avoid the use of BEVYXXA in patients receiving P-gp inducers.
7.3 Anticoagulants, Antiplatelets, and Thrombolytics
Co-administration of anticoagulants, antiplatelet drugs, and thrombolytics may increase the risk of bleeding. Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with anticoagulants, aspirin, other platelet aggregation inhibitors, and/or NSAIDs [see Warnings and Precautions (5.1)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with the use of BEVYXXA in pregnant women, but treatment is likely to increase the risk of hemorrhage during pregnancy and delivery (see Clinical Considerations). Betrixaban was studied in reproductive and developmental toxicology studies in rats and rabbits during the period of organogenesis at exposures up to 44 times the recommended clinical dose of 80 mg daily. Although betrixaban was not associated with adverse developmental fetal outcomes in animals, maternal toxicity (i.e., hemorrhage) was identified in these studies (see Data). BEVYXXA should be used during pregnancy only if the potential benefit outweighs the potential risk to the mother and fetus.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Embryo-fetal development studies were conducted in pregnant rats and rabbits during the period of organogenesis. In rats, no adverse embryo-fetal or teratogenic effects were seen when betrixaban was administered orally at doses up to 200 mg/kg/day, or 44 times the human dose of 80 mg/day when based on AUC. In rabbits, no adverse embryo-fetal or teratogenic effects were seen at doses up to 45 mg/kg/day, or 35 times the human exposure at a dose of 80 mg/day when based on AUC. Pregnant rabbits administered the highest dose of 150 mg/kg/day were terminated prematurely due to excessive maternal toxicities. Upon post-mortem examination, early and/or late resorptions and fetal deaths were observed at the 150 mg/kg dose, which may be linked to hemorrhage observed in various organs, including the reproductive tract.
In a rat pre-and-post-natal developmental study, betrixaban was administered orally during the period of organogenesis and through lactation Day 20 at doses up to 200 mg/kg/day. Maternal toxicities (including decreased body weight gain and food consumption and red/brown perivaginal substance) were observed at 200 mg/kg/day, which is approximately 44 times the human exposure when based on AUC. At a maternal dose up to 200 mg/kg/day, betrixaban did not have adverse effects on sexual maturation, reproductive performance, and behavioral development of the F1 generation.
8.2 Lactation
Risk Summary
No data are available regarding the presence of betrixaban or its metabolites in human milk, the effects of the drug on the breast-fed infant, or the effects of the drug on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BEVYXXA and any potential adverse effects on the breast-fed child from BEVYXXA or from the underlying maternal condition.
8.5 Geriatric Use
Of the total number of patients in the APEX clinical study, 90% were 65 years and over, while 68.6% were ≥ 75 years. No clinically significant differences in safety or effectiveness were observed between older and younger patients.
8.6 Renal Impairment
Patients with severe renal impairment (CrCl ≥ 15 to < 30 mL/min computed by Cockcroft-Gault using actual body weight) may have an increased risk of bleeding events. Reduce the BEVYXXA dose for patients with severe renal impairment. Monitor patients closely, and promptly evaluate any signs or symptoms of blood loss in these patients [see Dosage and Administration (2.3), Warnings and Precautions (5.3), Clinical Pharmacology (12.3)]. No dose adjustment is needed for mild or moderate renal impairment (CrCl > 30 mL/min, computed by Cockcroft-Gault using actual body weight).
8.7 Hepatic Impairment
In a pharmacokinetic study, compared to subjects with normal hepatic function, AUC increases of 30% were observed in subjects with mild hepatic impairment. No dose adjustment is required in patients with mild hepatic impairment.
Compared to subjects with normal hepatic function, AUC increases of 57% were observed in subjects with moderate hepatic impairment. The safety or pharmacokinetics (PK) of BEVYXXA in patients with severe hepatic impairment were not evaluated [see Clinical Pharmacology (12.3)]. Avoid the use of BEVYXXA in patients with moderate and severe hepatic impairment or with any hepatic disease associated with coagulopathy [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Overdose of BEVYXXA increases the risk of bleeding [see Warnings and Precautions (5.1)].
A specific reversal agent for BEVYXXA is not available. There is no experience with hemodialysis in individuals receiving betrixaban. Protamine sulfate, vitamin K, and tranexamic acid are not expected to reverse the anticoagulant activity of betrixaban.
11 DESCRIPTION
Betrixaban, a factor Xa (FXa) inhibitor, is chemically described as N-(5-chloropyridin-2-yl)-2-[4-(N,N-dimethylcarbamimidoyl)-benzoylamino]-5-methoxybenzamide in the form of maleate salt. The molecular formula of betrixaban maleate is C27H26ClN5O7, which corresponds to a molecular weight of 567.98. Betrixaban maleate has the following structural formula:
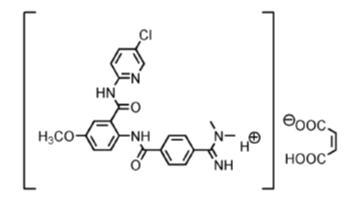
BEVYXXA capsules are available for oral administration in strengths of 40 mg and 80 mg of betrixaban, equivalent to 50.28 mg and 100.56 mg of betrixaban maleate, respectively. Each BEVYXXA capsule contains the following inactive ingredients: croscarmellose sodium, dextrose monohydrate, and magnesium stearate in a hard gelatin capsule.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Betrixaban is an oral FXa inhibitor that selectively blocks the active site of FXa and does not require a cofactor (such as Antithrombin III) for activity. Betrixaban inhibits free FXa and prothrombinase activity. By directly inhibiting FXa, betrixaban decreases thrombin generation (TG). Betrixaban has no direct effect on platelet aggregation.
12.2 Pharmacodynamics
Inhibition of FXa by betrixaban results in an inhibition of thrombin generation at clinically relevant concentrations, and the maximum inhibition of thrombin generation coincides with the time of peak betrixaban concentrations.
Cardiac Electrophysiology
In a study that evaluated the effect of betrixaban on the QT interval, a concentration-dependent increase in the QTc interval was observed. Based on the observed concentration-QTc relationship, a mean (upper 95% CI) QTc prolongation of 4 ms (5 ms) is predicted for 80 mg betrixaban and 13 ms (16 ms) for a 4.7-fold increase in exposure [see Clinical Pharmacology (12.3)].
12.3 Pharmacokinetics
Within the anticipated therapeutic dose range, a two-fold increase in dose resulted in a three-fold increase in exposure in the single ascending dose study. A two-fold increase in betrixaban exposure was observed after repeat dosing, and the time to steady-state is 6 days (without an initial loading dose).
Absorption
The oral bioavailability of betrixaban for an 80 mg dose is 34%, and peak concentrations occurred within 3 to 4 hours. Betrixaban is also a substrate of P-gp.
Effect of Food
When administered with a low-fat (900 calories, 20% fat) or high-fat (900 calories, 60% fat) meal, Cmax and AUC were reduced as compared to the fasting state by an average of 70% and 61% for low-fat and an average of 50% and 48% for high-fat, respectively. The effect of food on betrixaban PK could be observed for up to 6 hours after meal intake.
Distribution
The apparent volume of distribution is 32 L/kg. In vitro plasma protein binding is 60%.
Elimination
The effective half-life of betrixaban is 19 to 27 hours.
Metabolism
Unchanged betrixaban is the predominant component found in human plasma. Two inactive major metabolites formed by CYP-independent hydrolysis comprise the other components in plasma, accounting for 15 to 18% of the circulating drug-related material. Less than 1% of the minor metabolites could be formed via metabolism by the following CYP enzymes: 1A1, 1A2, 2B6, 2C9, 2C19, 2D6, and 3A4.
Excretion
Following oral administration of radio-labeled betrixaban, approximately 85% of the administered compound was recovered in the feces and 11% recovered in the urine. In a study of intravenous betrixaban, a median value of 17.8% of the absorbed dose was observed as unchanged betrixaban in urine.
Specific Populations
Male and Female Patients
No clinically significant changes in betrixaban PK were observed between males and females.
Patients with Renal Impairment
In a dedicated renal impairment study, mean AUC0-24 on Day 8 was increased by 1.89, 2.27, and 2.63-fold in mild (eGFRMDRD ≥ 60 to < 90 mL/min/1.73 m2), moderate (eGFRMDRD ≥ 30 to < 60 mL/min/1.73 m2), and severe (eGFRMDRD ≥ 15 to < 30 mL/min/1.73 m2) renal-impaired patients, respectively, compared to healthy volunteers [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
The safety and PK of single-dose BEVYXXA (80 mg) were evaluated in otherwise healthy subjects with varying degrees of hepatic impairment (i.e., normal, mild, and moderate subjects). The maximum and total betrixaban exposure, as measured by Cmax and AUC0-∞, were approximately 60% and 30% higher, respectively, for subjects with mild hepatic impairment compared to subjects with normal hepatic function. Betrixaban Cmax and AUC0-∞ were approximately 119% and 57% higher, respectively, for subjects with moderate hepatic impairment compared to subjects with normal hepatic function. Patients with severe hepatic impairment were not studied [see Use in Specific Populations (8.7)].
Drug Interaction Studies
The effects of coadministered drugs on the PK of betrixaban exposure based on drug interaction studies are summarized in Figure 1.
Figure 1: Effect of Coadministered Drugs on the Pharmacokinetics of Betrixaban
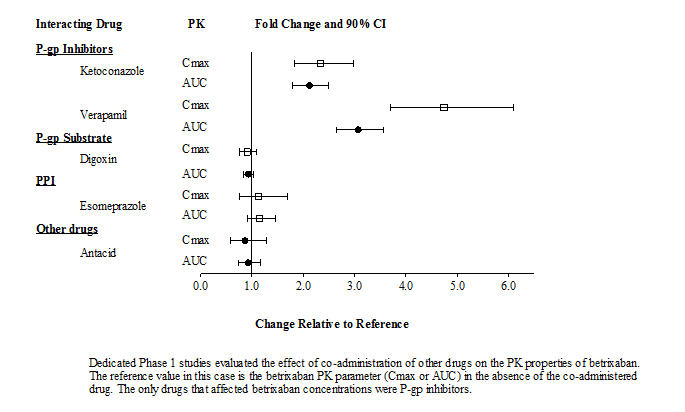
Dedicated Phase 1 studies evaluated the effect of co-administration of other drugs on the pharmacokinetic properties of betrixaban. The reference value in this case is the betrixaban pharmacokinetic parameter (Cmax or AUC) in the absence of the co-administered drug. The only drugs that affected betrixaban concentrations were P-gp inhibitors.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with betrixaban have not been performed.
Betrixaban was not mutagenic in bacteria (Ames-Test) or clastogenic in Chinese hamster ovary cells in vitro or in the rat micronucleus test in vivo.
In a study to assess fertility and early embryonic development to implantation, oral doses of betrixaban were administered to male and female rats. There was no evidence that betrixaban up to 150 mg/kg/day adversely affected male or female fertility, reproductive performance, or embryo-fetal viability.
14 CLINICAL STUDIES
The clinical evidence for the effectiveness of BEVYXXA is derived from the APEX clinical trial [NCT01583218]. APEX was a randomized, double-blind, multinational study comparing extended duration BEVYXXA (35 to 42 days) to short duration enoxaparin (6 to 14 days) in the prevention of VTE events in hospitalized, acutely medically ill patients with risk factors for VTE.
Eligible patients included adults who were at least 40 years of age, hospitalized for an acute medical illness, at risk for VTE due to moderate or severe immobility, and had additional risk factors for VTE (described below). Expected duration of hospitalization was at least 3 days, and patients were expected to be moderately or severely immobilized for at least 24 hours. The causes for hospitalization included heart failure, respiratory failure, infectious disease, rheumatic disease, or ischemic stroke. At study initiation, eligible patients were required to have one of the following additional risk factors for VTE:
- ≥ 75 years of age,
- 60 through 74 years of age with D-dimer ≥ 2 × ULN, or
- 40 through 59 years of age with D-dimer ≥ 2 × ULN and a history of either VTE or cancer.
A total of 7,513 patients were randomized 1:1 to:
- BEVYXXA arm (BEVYXXA 160 mg orally on Day 1, then 80 mg once daily for 35 to 42 days AND enoxaparin subcutaneous placebo once daily for 6 to 14 days),
OR - Enoxaparin arm (enoxaparin 40 mg subcutaneously once daily for 6 to 14 days AND BEVYXXA placebo orally once daily for 35 to 42 days).
Patients with severe renal impairment (creatinine clearance ≥ 15 and < 30 mL/min) received reduced doses of study medications (BEVYXXA 80 mg loading dose, then 40 mg once daily or enoxaparin 20 mg once daily) along with corresponding placebo.
Patients taking a concomitant P-gp inhibitor received BEVYXXA 80 mg loading dose, then 40 mg once daily or enoxaparin 40 mg subcutaneously once daily for 6 to 14 days along with corresponding placebo.
Baseline characteristics were balanced between the treatment groups. The population was 55% female, 93% White, 2% Black, 0.2% Asian, and 5% others. The most prevalent acute medical illness at hospitalization was acutely decompensated heart failure (45%), followed by acute infection without septic shock (29%), acute respiratory failure (12%), acute ischemic stroke (11%), and acute rheumatic disorders (3%). The mean and median ages were 76.4 and 77 years, respectively, with 68% of patients ≥ 75 years of age. 97% were severely immobilized at study entry, and 62% had D-dimer ≥ 2 × ULN.
While the APEX Study was ongoing (after 35% enrollment), the study was amended to ensure enrollment of patients ≥ 75 years of age or with D-dimer values ≥ 2 × ULN. The APEX trial excluded patients whose condition required prolonged anticoagulation (e.g., concurrent VTE, atrial fibrillation, or cardiac valve prosthesis), were at increased risk of bleeding, had liver dysfunction, were on dual antiplatelet therapy, or patients who had both severe renal insufficiency (CrCl 15-29 mL/min) and required the concomitant use of a P-gp inhibitor.
The efficacy of BEVYXXA was based upon the composite outcome of the occurrence of any of the following events up to the Day 35 visit:
- Asymptomatic proximal deep vein thrombosis (DVT) (detected by ultrasound),
- Symptomatic proximal or distal DVT,
- Non-fatal pulmonary embolism (PE), or
- VTE-related death.
Efficacy analyses were performed based on the modified Intent-to-Treat (mITT) population. The mITT population consisted of all patients who had taken at least one dose of study drug and who had follow-up assessment data on one or more primary or secondary efficacy outcome components. A total of 7,441 patients (N=3,721 for BEVYXXA and N=3,720 for enoxaparin) was included in the mITT population.
The efficacy results for the APEX trial are provided in Table 5.
| BEVYXXA N=3,721 n (%) * | Enoxaparin N=3,720 n (%) * | Relative Risk (95% CI) † |
|
|---|---|---|---|
|
|||
| Composite Outcome | 165 (4.4) | 223 (6.0) | 0.75 (0.61, 0.91) |
| Asymptomatic Event | 133 (3.6) | 176 (4.7) | |
| Symptomatic DVT | 14 (0.4) | 22 (0.6) | |
| Non-fatal PE | 9 (0.2) | 18 (0.5) | |
| VTE-related Death | 13 (0.3) | 17 (0.5) | |
| Symptomatic Events ‡ | 35 (0.9) | 54 (1.5) | 0.64 (0.42, 0.98) |
For patients with D-dimer ≥ 2 × ULN at baseline, the event rate is 5.7% in the BEVYXXA arm versus 7.2% in the enoxaparin arm (relative risk = 0.79, 95% CI [0.63, 0.98]).
For patients with D-dimer ≥ 2 × ULN at baseline or age ≥ 75 years, the event rate is 4.7% in the BEVYXXA arm versus 6.0% in the enoxaparin arm (relative risk = 0.78, 95% CI [0.64, 0.96]).
Results for the primary efficacy analysis for subjects that were stratified at randomization to the 80 mg BEVYXXA dose group in the mITT population are shown in Table 6.
Patients who were randomized to receive 40 mg of BEVYXXA (those with severe renal impairment or receiving P-gp inhibitors) had VTE rates similar to the enoxaparin arm (6 to 14 days followed by placebo) shown in Table 7.
| BEVYXXA N=2,878 n (%) * | Enoxaparin N=2,926 n (%) * | Relative Risk (95% CI) † |
|
|---|---|---|---|
|
|||
| Composite Outcome | 120 (4.2) | 180 (6.2) | 0.68 (0.55, 0.86) |
| Asymptomatic Event | 100 (3.5) | 146 (5.0) | |
| Symptomatic DVT | 11 (0.4) | 17 (0.6) | |
| Non-fatal PE | 4 (0.1) | 14 (0.5) | |
| VTE-related Death | 8 (0.3) | 12 (0.4) | |
| Symptomatic Events ‡ | 22 (0.8) | 41 (1.4) | 0.55 (0.33, 0.92) |
| Severe Renal Impairment | Concomitant Use of P-gp Inhibitor | |||||
|---|---|---|---|---|---|---|
| BEVYXXA N=174 n (%) * | Enoxaparin N=149 n (%) * | Relative Risk (95% CI) † | BEVYXXA N=669 n (%) * | Enoxaparin N=645 n (%) * | Relative Risk (95% CI) † |
|
|
||||||
| Composite Outcome | 12 (6.9) | 10 (6.7) | 1.0 (0.45, 2.23) | 33 (4.9) | 33 (5.1) | 1.0 (0.63, 1.60) |
| Asymptomatic Event | 9 (5.2) | 7 (4.7) | 24 (3.6) | 23 (3.6) | ||
| Symptomatic DVT | 0 | 1 (0.7) | 3 (0.4) | 4 (0.6) | ||
| Non-fatal PE | 2 (1.1) | 2 (1.3) | 3 (0.4) | 2 (0.3) | ||
| VTE-related Death | 2 (1.1) | 0 | 3 (0.4) | 5 (0.8) | ||
| Symptomatic Events ‡ | 4 (2.3) | 3 (2.0) | 9 (1.3) | 10 (1.6) | ||
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
BEVYXXA 40 mg (size 4) capsules have a light grey body with 40 printed in black and a light blue cap with PTLA printed in white.
- Bottles of 100 (NDC 69853-0202-1)
BEVYXXA 80 mg (size 2) capsules have a light grey body with 80 printed in black and a blue cap with PTLA printed in white.
- Bottles of 100 (NDC 69853-0201-1)
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risk of Bleeding
Advise patients that it might take longer than usual for bleeding to stop, and that they may bruise or bleed more easily when treated with BEVYXXA. Instruct patients to report any unusual bleeding to their physician [see Warnings and Precautions (5.1)].
Instruct patients to tell their physicians and dentists that they are taking BEVYXXA, and/or any other products known to affect bleeding (including nonprescription products, such as aspirin or NSAIDs), before any surgery or medical or dental procedure is scheduled and before any new drug is taken [see Warnings and Precautions (5.1, 5.4)].
Use in Patients with Severe Renal Impairment
Advise patients that the risk of bleeding is higher in people who have severe kidney problems (severe renal impairment) [see Warnings and Precautions (5.3)].
Spinal/Epidural Hematoma
Advise patients having neuraxial anesthesia or spinal puncture to watch for signs and symptoms of spinal or epidural hematomas, such as numbness or weakness of the legs, or bowel or bladder dysfunction [see Warnings and Precautions (5.2)]. Instruct patients to contact their physician immediately if any of these symptoms occur.
Pregnancy and Lactation
Advise female patients to inform their physicians if they are pregnant or plan to become pregnant or are breastfeeding or intend to breastfeed during treatment with BEVYXXA [see Use in Specific Populations (8.1, 8.2)].
How to Take BEVYXXA
Instruct patients to take BEVYXXA with food, and instruct patients on what to do if a dose is missed [see Dosage and Administration (2.1, 2.5)].
Manufactured for:
Portola Pharmaceuticals LLC
South San Francisco, California 94080, USA
BTX-US-V.3.0
| MEDICATION GUIDE BEVYXXA® (BEV vix a) capsules |
|
|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Issued: 6/2017 |
| What is the most important information I should know about BEVYXXA? BEVYXXA can cause serious side effects, including:
|
|
| What is BEVYXXA?
BEVYXXA is a prescription medicine used to help prevent blood clots in adults who are hospitalized for an acute illness, and are at risk of getting blood clots because of the loss of or decreased ability to move around (mobility) and other risks for getting blood clots.
|
|
Do not take BEVYXXA if you:
|
|
Before taking BEVYXXA, tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some of your other medicines may affect the way BEVYXXA works. Certain medicines may increase your risk of bleeding when taken with BEVYXXA. See "What is the most important information I should know about BEVYXXA?" and "What is BEVYXXA?" |
|
How should I take BEVYXXA?
|
|
| What are the possible side effects of BEVYXXA? BEVYXXA can cause serious side effects. The most common side effect of BEVYXXA is bleeding. These are not all of the side effects of BEVYXXA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should I store BEVYXXA?
|
|
| General information about the safe and effective use of BEVYXXA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BEVYXXA for a condition for which it was not prescribed. Do not give BEVYXXA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about BEVYXXA that is written for health professionals. |
|
| What are the ingredients in BEVYXXA?
Active ingredient: betrixaban Inactive ingredients: dextrose monohydrate, croscarmellose sodium, magnesium stearate, and a hard gelatin capsule Manufactured for: Portola Pharmaceuticals LLC, South San Francisco, California 94080 USA, Copyright© Portola Pharmaceuticals. For more information, call 1-866-777-5947 or go to www.BEVYXXA.com |
|
BTX-US-MG-V.1.3
| BEVYXXA
betrixaban capsule, gelatin coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| BEVYXXA
betrixaban capsule, gelatin coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Portola Pharmaceuticals LLC (142296016) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon, Inc. | 240769596 | MANUFACTURE(69853-0202, 69853-0201) , ANALYSIS(69853-0202, 69853-0201) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc. | 053217022 | LABEL(69853-0202, 69853-0201) , PACK(69853-0202, 69853-0201) | |

