Label: NEXT CHOICE- levonorgestrel tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-443-02 - Packager: Rebel Distributors Corp.
- This is a repackaged label.
- Source NDC Code(s): 52544-475
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 1, 2009
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION

PHYSICIAN LABELING
Levonorgestrel Tablets, 0.75 mg
Rx only for women age 17 and younger
This product is not approved for nonprescription use.
Levonorgestrel tablets are a prescription–only emergency contraceptive for women age 17 and younger. This product is not approved for nonprescription use. Levonorgestrel tablets are intended to prevent pregnancy after known or suspected contraceptive failure or unprotected intercourse. Emergency contraceptive pills (like all oral contraceptives) do not protect against infection with HIV (the virus that causes AIDS) and other sexually transmitted diseases.
-
DESCRIPTION
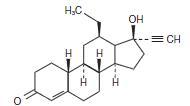
Emergency contraceptive tablet. Each levonorgestrel tablet contains 0.75 mg of a single active steroid ingredient, levonorgestrel [18,19-Dinorpregn-4-en-20-yn-3-one-13-ethyl-17-hydroxy-, (17α)-(-)-], a totally synthetic progestogen. The inactive ingredients present are colloidal silicon dioxide, corn starch, FD&C Yellow #6, magnesium stearate, povidone, and lactose monohydrate. Levonorgestrel has a molecular weight of 312.45, and the following structural and molecular formulas:
C21H28O2
-
CLINICAL PHARMACOLOGY
Emergency contraceptives are not effective if the woman is already pregnant. Levonorgestrel tablets are believed to act as an emergency contraceptive principally by preventing ovulation or fertilization (by altering tubal transport of sperm and/or ova). In addition, they may inhibit implantation (by altering the endometrium). They are not effective once the process of implantation has begun.
Pharmacokinetics
Absorption
No specific investigation of the absolute bioavailability of levonorgestrel tablets in humans has been conducted. However, literature indicates that levonorgestrel is rapidly and completely absorbed after oral administration (bioavailability about 100%) and is not subject to first pass metabolism. After a single dose of levonorgestrel tablets (0.75 mg) administered to 16 women under fasting conditions, maximum serum concentrations of levonorgestrel are 14.1 ± 7.7 ng/mL (mean ± SD) at an average of 1.6 ± 0.7 hours. No formal study of the effect of food on the absorption of levonorgestrel has been undertaken.
Table 1 Pharmacokinetic Parameter Values Following Single Dose Administration of Levonorgestrel Tablets, 0.75 mg to Healthy Female Volunteers Mean (± S.D.) N
Cmax
(ng/mL)Tmax
(h)CL
(L/h)Vd
(L)T1/2
(h)AUC0-∞
(ng/mL/h)16 14.1 ± 7.7 1.6 ± 0.7 7.7 ± 2.7 260.0 24.4 ± 5.3 123.1 ± 50.1 Distribution
Levonorgestrel in serum is primarily protein bound. Approximately 50% is bound to albumin and 47.5% is bound to sex hormone binding globulin (SHBG).
Metabolism
Following a single oral dosage, levonorgestrel does not appear to be extensively metabolized by the liver. The primary metabolites are 3α,5β- and 3α,5α-tetrahydrolevonorgestrel with 16β-hydroxynorgestrel also identified. Together, these account for less than 10% of parent plasma levels. Urinary metabolites hydroxylated at the 2α and 16β positions have also been identified. Small amounts of the metabolites are present in plasma as sulfate and glucuronide conjugates.
Excretion
The elimination half-life of levonorgestrel following single dose administration as levonorgestrel tablets (0.75 mg) is 24.4 ± 5.3 hours. Excretion following single dose administration as emergency contraception is unknown, but based on chronic, low-dose contraceptive use, levonorgestrel and its metabolites are primarily excreted in the urine, with smaller amounts recovered in the feces.
SPECIAL POPULATIONS
Geriatric
This product is not intended for use in geriatric (age 65 years or older) populations and pharmacokinetic data are not available for this population.
Pediatric
This product is not intended for use in pediatric (premenarcheal) populations, and pharmacokinetic data are not available for this population.
Race
No formal studies have evaluated the effect of race. However, clinical trials demonstrated a higher pregnancy rate in the Chinese population with both levonorgestrel tablets and the Yuzpe regimen (another form of emergency contraception consisting of two doses of ethinyl estradiol 0.1 mg + levonorgestrel 0.5 mg). The reason for this apparent increase in the pregnancy rate of emergency contraceptives in Chinese women is unknown.
Hepatic Insufficiency and Renal Insufficiency
No formal studies have evaluated the effect of hepatic insufficiency or renal insufficiency on the disposition of emergency contraceptive tablets.
Drug-Drug Interactions
No formal studies of drug-drug interactions were conducted.
-
INDICATIONS AND USAGE
Levonorgestrel tablets are a prescription-only emergency contraceptive, for women age 17 and younger, that can be used to prevent pregnancy following unprotected intercourse or a known or suspected contraceptive failure. This product is not approved for nonprescription use.
To obtain optimal efficacy, the first tablet should be taken as soon as possible within 72 hours of intercourse. The second tablet must be taken 12 hours later.
-
Clinical Studies
A double-blind, controlled clinical trial in 1,955 evaluable women compared the efficacy and safety of levonorgestrel tablets, (one 0.75 mg tablet of levonorgestrel taken within 72 hours of intercourse, and one tablet taken 12 hours later) to the Yuzpe regimen (two tablets of 0.25 mg levonorgestrel and 0.05 mg ethinyl estradiol, taken within 72 hours of intercourse, and two tablets taken 12 hours later). Levonorgestrel tablets, were at least as effective as the Yuzpe regimen in preventing pregnancy. After a single act of intercourse, the expected pregnancy rate of 8% (with no contraception) was reduced to approximately 1% with levonorgestrel tablets.
Emergency contraceptives are not as effective as routine contraception since their failure rate, while low based on a single use, would accumulate over time with repeated use (see WARNINGS). See Table 2 below.
Table 2 Percentage of women experiencing an unintended pregnancy during the first year of typical use and the first year of perfect use of contraception, and the percentage continuing use at the end of the first year, United States. % of Women Experiencing an Unintended Pregnancy within the First Year of Use % of Women Continuing Use at One Year3 Method (1) Tpical Use1 (2) Perfect Use2 (3) (4) Source: Trussell J, Contraceptive efficacy. In Hatcher RA, Trussell J, Stewart F, Cates W, Stewart GK, Kowal D, Guest F, Contraceptive Technology: Seventeenth Revised Edition. New York, NY: Irvington Publishers, 1998. 1 Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an unintended pregnancy during the first year if they do not stop use for any other reason. 2 Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an unintended pregnancy during the first year if they do not stop use for any other reason. 3 Among couples attempting to avoid pregnancy, the percentage who continue to use a method for one year. 4 The percentages of women becoming pregnant in columns (2) and (3) are based on data from populations where contraception is not used and from women who cease using contraception in order to become pregnant. Among such populations, about 89% become pregnant within one year. This estimate was lowered slightly (to 85%) to represent the percentage who would become pregnant within one year among women now relying on reversible methods of contraception if they abandoned contraception altogether. 5 Foams, creams, gels, vaginal suppositories and vaginal film. 6 Cervical mucus (ovulation) method supplemented by calendar in the preovulatory and basal body temperature in the post-ovulatory phases. 7 With spermicidal cream or jelly. 8 Without spermicides. 9 The treatment schedule is one dose within 72 hours after unprotected intercourse and a second dose 12 hours after the first dose. The Food and Drug Administration has declared the following brands of oral contraceptives to be safe and effective for emergency contraception: Ovral®* (1 dose is 2 white pills), Alesse®* (1 dose is 5 pink pills), Nordette®* or Levlen®* (1 dose is 2 light-orange pills), Lo/Ovral®* (1 dose is 4 white pills), Triphasil®* or Tri-Levlen®* (1 dose is 4 yellow pills). 10 However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feeds are introduced or the baby reaches six months of age. Chance4 85 85 Spermicide5 26 6 40 Periodic Abstinence 25 63 Calendar 9 Ovulation Method 3 Sympto-thermal6 2 Post-ovulation 1 Withdrawal 19 4 Cap7 Parous Women 40 26 42 Nulliparous Women 20 9 56 Sponge Parous Women 40 20 42 Nulliparous Women 20 9 56 Diaphragm7 20 6 56 Condom8 Female (Reality®*) 21 5 56 Male 14 3 61 Pill 5 71 Progestin Only 0.5 Combined 0.1 0.1 IUD Progesterone T 2.0 1.5 81 Copper T 380A 0.8 0.6 78 LNg 20 0.1 0.1 81 Depo-Provera®* 0.3 0.3 70 Norplant®* and
Norplant-2®*
0.05
0.05
88Female Sterilization 0.5 0.5 100 Male Sterilization 0.15 0.10 100 Emergency Contraceptive Pills: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 75%.9 Lactational Amenorrhea Method: LAM is a highly effective, temporary, method of contraception.10 -
CONTRAINDICATIONS
Progestin-only contraceptive pills (POPs) are used as a routine method of birth control over longer periods of time, and are contraindicated in some conditions. It is not known whether these same conditions apply to the levonorgestrel tablets regimen consisting of the emergency use of two progestin pills. POPs however, are not recommended for use in the following conditions:
-
Known or suspected pregnancy
-
Hypersensitivity to any component of the product
-
-
WARNINGS
Levonorgestrel tablets are not recommended for routine use as a contraceptive.
Levonorgestrel tablets are not effective in terminating an existing pregnancy.Effects on Menses
Menstrual bleeding patterns are often irregular among women using progestin-only oral contraceptives and in clinical studies of levonorgestrel for postcoital and emergency contraceptive use. Some women may experience spotting a few days after taking levonorgestrel tablets. At the time of expected menses, approximately 75% of women using levonorgestrel tablets had vaginal bleeding similar to their normal menses, 12% to 13% bled more than usual, and 12% bled less than usual. The majority of women (87%) had their next menstrual period at the expected time or within ± 7 days, while 13% had a delay of more than 7 days beyond the anticipated onset of menses. If there is a delay in the onset of menses beyond 1 week, the possibility of pregnancy should be considered.
Ectopic Pregnancy
Ectopic pregnancies account for approximately 2% of reported pregnancies (19.7 per 1,000 reported pregnancies). Up to 10% of pregnancies reported in clinical studies of routine use of progestin-only contraceptives are ectopic. A history of ectopic pregnancy need not be considered a contraindication to use of this emergency contraceptive method. Health providers, however, should be alert to the possibility of an ectopic pregnancy in women who become pregnant or complain of ower abdominal pain after taking levonorgestrel tablets.
-
PRECAUTIONS
Pregnancy
Many studies have found no effects on fetal development associated with long-term use of contraceptive doses of oral progestins (POPs). The few studies of infant growth and development that have been conducted with POPs have not demonstrated significant adverse effects.
STD/HIV
Levonorgestrel tablets, like progestin-only contraceptives, do not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Physical Examination and Follow-up
A physical examination is not required prior to prescribing levonorgestrel tablets. A follow-up physical or pelvic examination, however, is recommended if there is any doubt concerning the general health or pregnancy status of any woman after taking levonorgestrel tablets.
Carbohydrate Metabolism
The effects of levonorgestrel tablets on carbohydrate metabolism are unknown. Some users of progestin-only oral contraceptives (POPs) may experience slight deterioration in glucose tolerance, with increases in plasma insulin; however, women with diabetes mellitus who use POPs do not generally experience changes in their insulin requirements. Nonetheless, diabetic women should be monitored while taking levonorgestrel tablets.
Drug Interactions
Theoretically, the effectiveness of low-dose progestin-only pills is reduced by hepatic enzyme-inducing drugs such as the anticonvulsants phenytoin, carbamazepine, and barbiturates, and the antituberculosis drug rifampin. No significant interaction has been found with broad-spectrum antibiotics. It is not known whether the efficacy of levonorgestrel tablets would be affected by these or any other medications.
Nursing Mothers
Small amounts of progestin pass into the breast milk in women taking progestinonly pills for long-term contraception resulting in steroid levels in infant plasma of 1% to 6% of the levels of maternal plasma. However, no adverse effects due to progestin-only pills have been found on breastfeeding performance, either in the quality or quantity of the milk, or on the health, growth or development of the infant.
Pediatric Use
Safety and efficacy of progestin-only pills have been established in women of reproductive age for long-term contraception. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and for users 16 years and older. Use of levonorgestrel tablets emergency contraception before menarche is not indicated.
-
ADVERSE REACTIONS
The most common adverse events in the clinical trial for women receiving levonorgestrel tablets included nausea (23%), abdominal pain (18%), fatigue (17%), headache (17%), and menstrual changes. The table below shows those adverse events that occurred in ≥5% of levonorgestrel tablets users.
Table 3 Adverse Events in ≥5% of Women, by % Frequency Most Common Adverse Events Levonorgestrel
N=977 (%)Nausea 23.1 Abdominal Pain 17.6 Fatigue 16.9 Headache 16.8 Heavier Menstrual Bleeding 13.8 Lighter Menstrual Bleeding 12.5 Dizziness 11.2 Breast Tenderness 10.7 Other complaints 9.7 Vomiting 5.6 Diarrhea 5.0 Levonorgestrel tablets demonstrated a superior safety profile over the Yuzpe regimen for the following adverse events:
-
Nausea: Occurred in 23% of women taking levonorgestrel tablets (compared to 50% with Yuzpe)
-
Vomiting: Occurred in 6% of women taking levonorgestrel tablets (compared to 19% with Yuzpe)
-
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
One levonorgestrel tablet should be taken orally as soon as possible within 72 hours after unprotected intercourse. The second tablet should be taken 12 hours after the first dose. Efficacy is better if levonorgestrel tablets are taken as directed as soon as possible after unprotected intercourse. Levonorgestrel tablets can be used at any time during the menstrual cycle.
The user should be instructed that if she vomits within one hour of taking either dose of medication she should contact her health care professional to discuss whether to repeat that dose.
-
HOW SUPPLIED
Levonorgestrel Tablets, 0.75 mg are available for a single course of treatment in PVC/aluminum foil blister packages of two tablets each. Each tablet is peach, round, bevel edged, and flat faced embossed with “475” on one side and “WATSON” on the other side.
Available as:
Unit-of-use NDC 0591-0475-36
Store levonorgestrel tablets at controlled room temperature, 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [See USP].
*The following are registered trademarks of their respective manufacturers: Reality® is manufactured by Female Health Company and a registered trademark of Meijer, Inc.; Depo-Provera® is manufactured by and a registered trademark of Pharmacia and Upjohn; Norplant® is manufactured by and a registered trademark of Population Council; Ovral®, Alesse®, Triphasil® and Lo Ovral®/Wyeth Pharmaceuticals, Inc.; Nordette®/Duramed Pharmaceuticals, Inc.; Levlen® and Tri- Levlen®/Bayer Healthcare.
Watson Laboratories, Inc.
Corona, CA 92880 USA
Phone: 1-800-272-5525
14816-3Repackaged by:
Rebel Distributors Corp.
Thousand Oaks, CA 91320
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEXT CHOICE
levonorgestrel tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-443(NDC:52544-475) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVONORGESTREL (UNII: 5W7SIA7YZW) (LEVONORGESTREL - UNII:5W7SIA7YZW) LEVONORGESTREL 0.75 mg Inactive Ingredients Ingredient Name Strength COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE (UNII: FZ989GH94E) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Product Characteristics Color ORANGE (peach) Score no score Shape ROUND (bevel edged, flat faced) Size 6mm Flavor Imprint Code 475;WATSON Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-443-02 1 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078666 07/21/2009 Labeler - Rebel Distributors Corp. (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp. 118802834 relabel, repack


