ZICAM COLD REMEDY ORAL MIST- zinc acetate and zinc gluconate spray
Matrixx Initiatives, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Zicam® Cold Remedy Oral Mist™
Uses
- reduces duration of the common cold
- helps reduce severity of cold symptoms:
- sore throat
- stuffy nose
- sneezing
- coughing
- nasal congestion
Zicam® Cold Remedy was formulated to shorten the duration of the common cold and was not formulated to be effective for flu or allergies.
Warnings
For oral use only
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
When using this product avoid contact with eyes. Rinse right away with water if it gets in eyes and seek medical help right away.
Directions
- for best results, use at the first sign of a cold and continue to use until symptoms completely subside
- adults and children 12 years of age and older:
- spray 4 times in mouth at the onset of symptoms. Spray on inside of cheeks, roof of mouth and gums. Retain for 15 seconds. Swallow.
- repeat every 2- 3 hours, not to exceed 7 doses in 24 hours. Take until symptoms are gone.
- to avoid minor stomach upset, do not take on an empty stomach
- do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
- children under 12 years of age: ask a doctor before use
Inactive ingredients
benzalkonium chloride, glycerin, menthol, natural & artificial flavor, PEG-40 hydrogenated castor oil, purified water, sucralose, xylitol
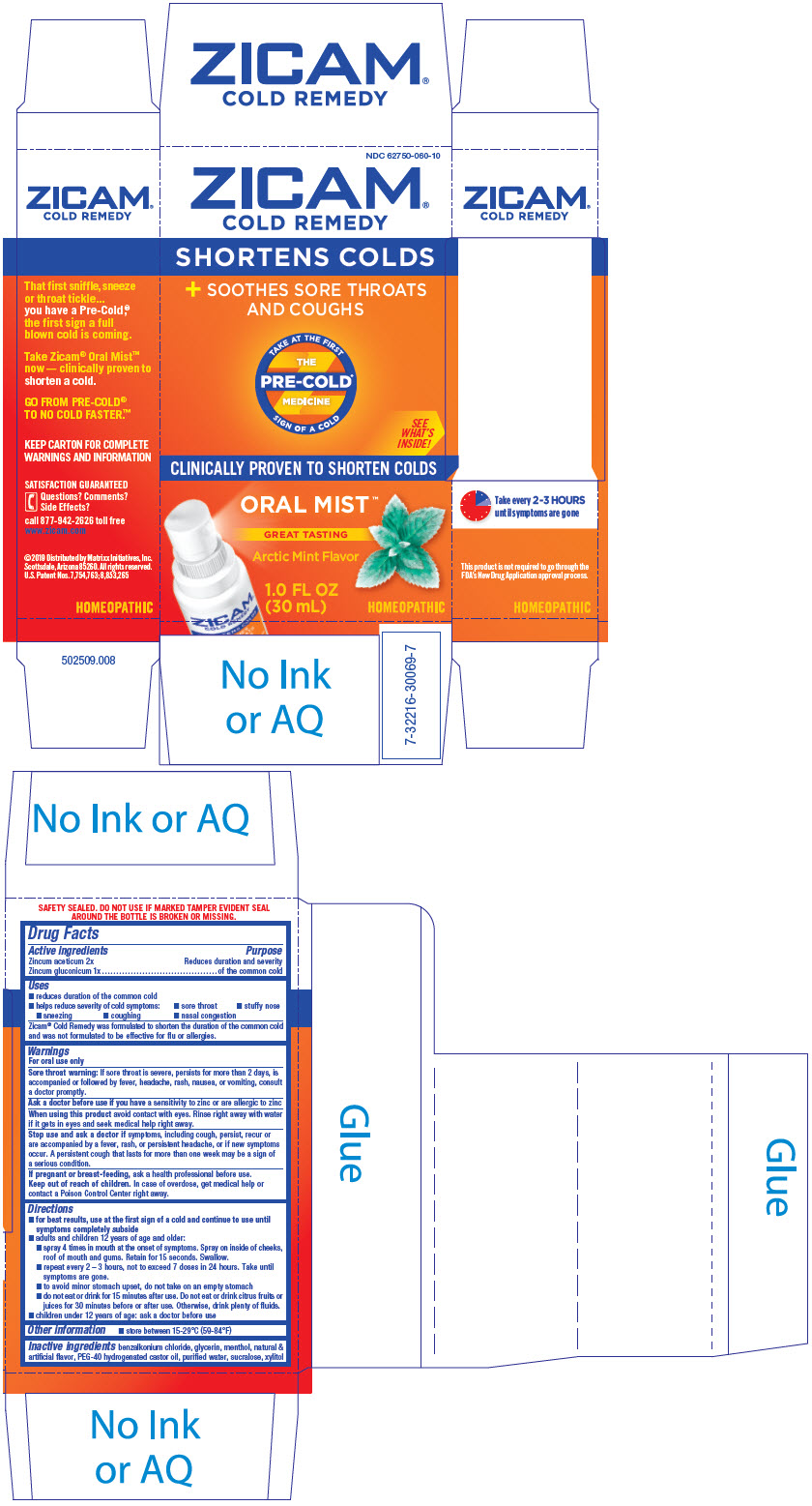
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
NDC 62750-060-10
ZICAM®
COLD REMEDY
SHORTENS COLDS
+ SOOTHES SORE THROATS
AND COUGHS
TAKE AT THE FIRST
SIGN OF A COLD
THE
PRE-COLD®
MEDICINE
SEE
WHAT'S
INSIDE!
CLINICALLY PROVEN TO SHORTEN COLDS
ORAL MIST™
GREAT TASTING
Arctic Mint Flavor
1.0 FL OZ
(30 mL)
HOMEOPATHIC
| ZICAM COLD REMEDY ORAL MIST
zinc acetate and zinc gluconate spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Matrixx Initiatives, Inc. (790037253) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Accupac, Inc | 071609663 | MANUFACTURE(62750-060) | |