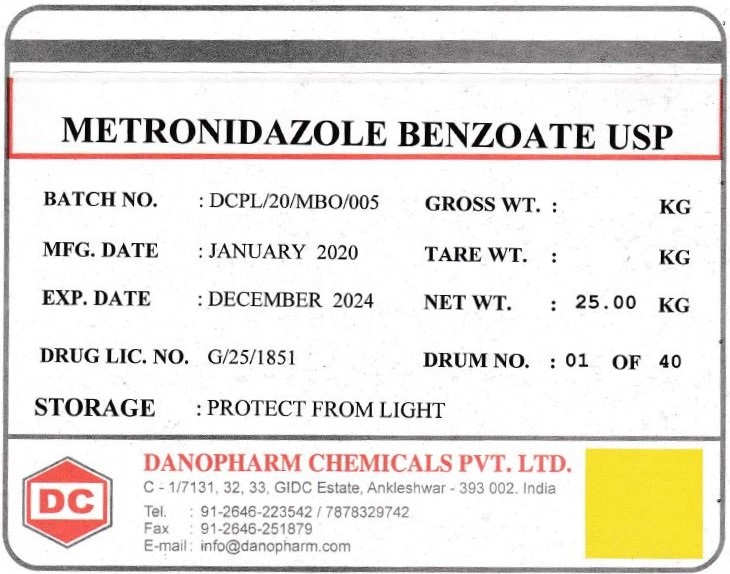
METRONIDAZOLE BENZOATE- metronidazole benzoate powder
Danopharm Chemicals
----------
Metronidazole Benzoate
| METRONIDAZOLE BENZOATE
metronidazole benzoate powder |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Danopharm Chemicals (725030519) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Danopharm Chemicals Pvt. Ltd. | 862811935 | manufacture | |
Revised: 8/2022
Document Id: 2e0817bc-8e05-45e4-9759-849bf40a8c73
Set id: ea8bde75-8080-43a3-99a9-cfa56dce47a5
Version: 3
Effective Time: 20220818
Danopharm Chemicals