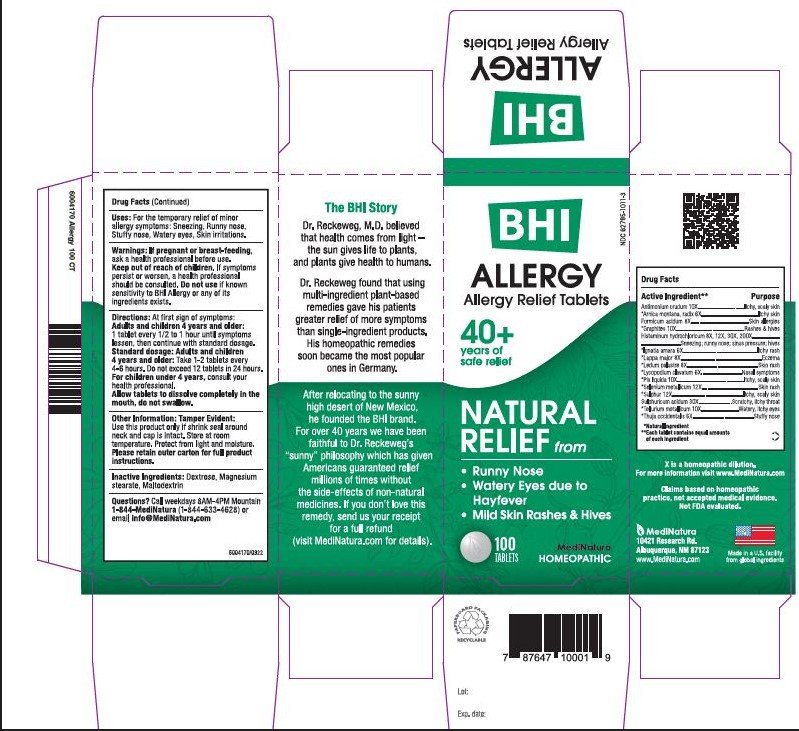
Label: BHI ALLERGY- antimony trisulfide, arnica montana root, formic acid, graphite, histamine dihydrochloride,strychnos ignatii seed, arctium lappa root, ledum palustre twig, lycopodium clavatum spore, pine tar, selenium, sulfur, sulfuric acid, tellurium, thuja occidentalis leafy twig tablet
- NDC Code(s): 62795-1011-2, 62795-1011-3
- Packager: MediNatura
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated September 28, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- KEEP OUT OF REACH OF CHILDREN
- INDICATION AND USAGE
- WARNINGS
-
Directions
At first sign of symptoms: Adults and children 4 years and older:
1 tablet every 1/2 to 1 hour until symptoms lessen, then continue with standard dosage.
Standard dosage: Adults and children 4 years and older:
Take 1-2 tablets every 4 to 6 hours. Do not exceed 12 tablets in 24 hours.
For children under 4, consult your health professional.
Allow tablets to dissolve completely in the mouth, do not swallow.
-
ACTIVE INGREDIENTS
Active Ingredients:
Each tablet contains: Antimonium crudum 10X, *Arnica montana, radix 6X, Formicumacidum 8X, Graphites 10X, Histaminum hydrochloricum 8X, 12X, 30X, 200X, *Ignatia amara 6X, *Lappa major 8X, *Ledum palustre 8X, *Lycopodium clavatum 6X, *Pix liquida 10X, Selenium metallicum 12X, *Sulphur 12X, Sulphuricum acidum 30X, *Tellurium metallicum 10X, *Thuja occidentalis 6X 16.7 mg each.
*Natural ingredients
- INACTIVE INGREDIENTS
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BHI ALLERGY
antimony trisulfide, arnica montana root, formic acid, graphite, histamine dihydrochloride,strychnos ignatii seed, arctium lappa root, ledum palustre twig, lycopodium clavatum spore, pine tar, selenium, sulfur, sulfuric acid, tellurium, thuja occidentalis leafy twig tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62795-1011 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIMONY TRISULFIDE (UNII: F79059A38U) (ANTIMONY TRISULFIDE - UNII:F79059A38U) ANTIMONY TRISULFIDE 10 [hp_X] ARNICA MONTANA ROOT (UNII: MUE8Y11327) (ARNICA MONTANA ROOT - UNII:MUE8Y11327) ARNICA MONTANA ROOT 6 [hp_X] FORMIC ACID (UNII: 0YIW783RG1) (FORMIC ACID - UNII:0YIW783RG1) FORMIC ACID 8 [hp_X] GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 10 [hp_X] HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 8 [hp_X] STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 6 [hp_X] ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) (ARCTIUM LAPPA ROOT - UNII:597E9BI3Z3) ARCTIUM LAPPA ROOT 8 [hp_X] LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 8 [hp_X] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 6 [hp_X] PINE TAR (UNII: YFH4WC535J) (PINE TAR - UNII:YFH4WC535J) PINE TAR 10 [hp_X] SELENIUM (UNII: H6241UJ22B) (SELENIUM - UNII:H6241UJ22B) SELENIUM 12 [hp_X] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] SULFURIC ACID (UNII: O40UQP6WCF) (SULFURIC ACID - UNII:O40UQP6WCF) SULFURIC ACID 30 [hp_X] TELLURIUM (UNII: NQA0O090ZJ) (TELLURIUM - UNII:NQA0O090ZJ) TELLURIUM 10 [hp_X] THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 6 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) DEXTROSE (UNII: IY9XDZ35W2) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code Leafman Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62795-1011-3 1 in 1 CARTON 08/30/2014 1 100 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:62795-1011-2 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/11/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/30/2014 Labeler - MediNatura (079324099) Establishment Name Address ID/FEI Business Operations MediNatura 102783016 manufacture(62795-1011)