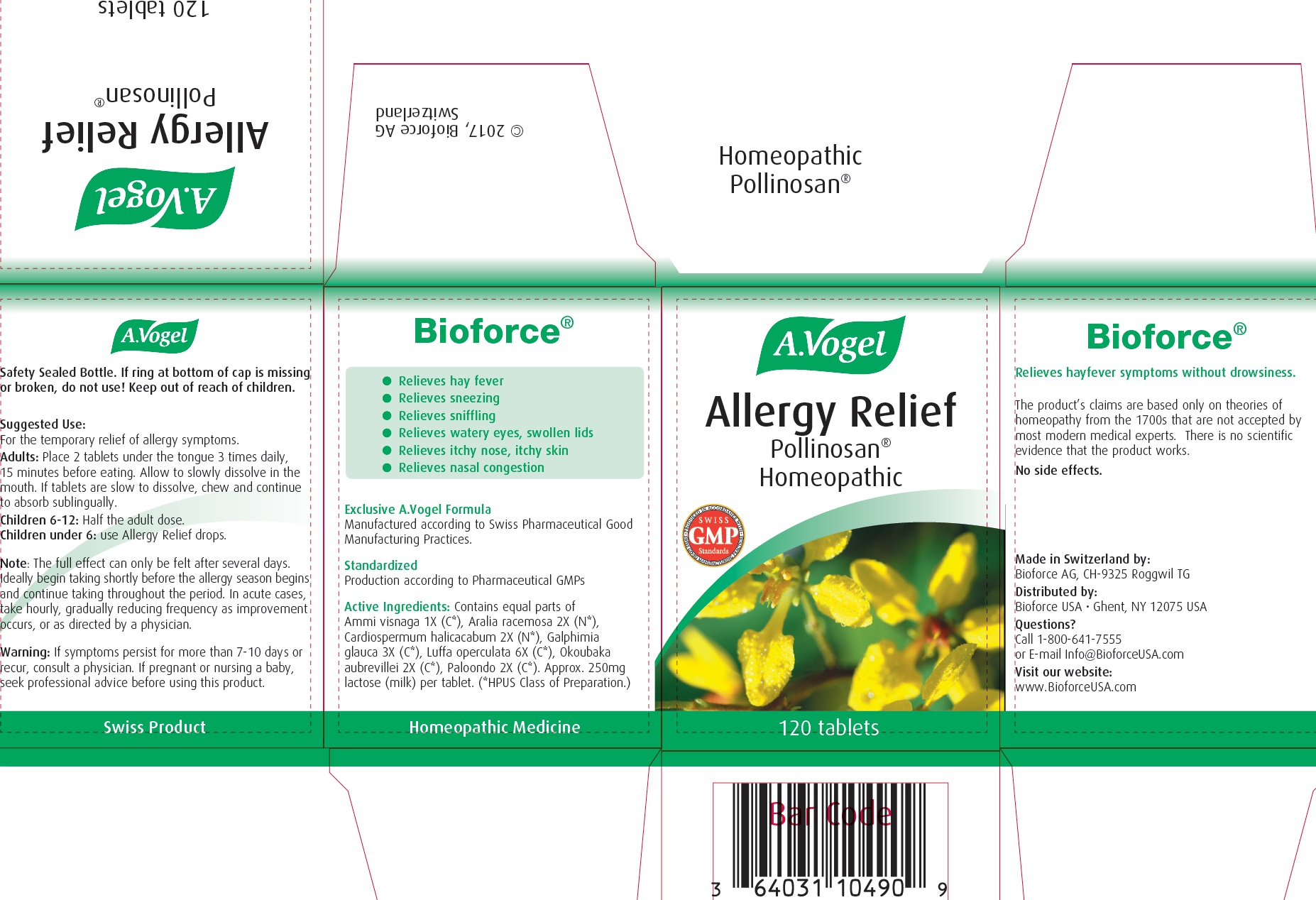
ALLERGY RELIEF HOMEOPATHIC POLLINOSAN- ammi visnaga, aralia racemosa, cardiospermum halicacabum, galphimia glauca, luffa operculata, okoubaka aubrevillei, paloondo tablet
Bioforce AG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Allergy Relief Homeopathic Pollinosan
Adults:
Place 2 tablets under the tongue 3 times daily, 15 minutes before eating. Allow to slowly dissolve in the mouth. If tablets are slow to dissolve, chew and continue to absorb sublingually.
Children 6-12: Half the adult dose.
Children under 6: use Allergy Relief drops.
Note:
The full effect can only be felt after several days. deally beign taking shortly before the allergy season begins and continue taking throughout the period. In acute cases, take hourly, gradually reducing frequency as improvement occurs, or as directed by a physician.
Warnings:
If symptoms persist for more than 7-10 days or recur, consult a physician. If pregnant or nursing a baby, seek professional advice before using this product.
- Relieves hay fever
- Relieves sneezing
- Relieves sniffling
- Relieves watery eyes, swollen lids
- Relieves itchy nose, itchy skin
- Relieves nasal congestion
Exclusive A.Vogel Formula
Manufactured according to Swiss Pharmaceutical Good Manufacturing Practices.
Active Ingredients :
Contains equal parts of Ammi visnaga 1X (C *), Aralia racemosa 2X (N *), Cardiospermum Halicacabum 2X (N *), Galphimia glauca 3X (C *), Luffa operculata 6X (C *), Okoubaka aubrevillei 2X (C *), Paloondo 2X (C *). Approx. 250mg lactose (milk) per tablet. ( *HPUS Class of Preparation.)
| ALLERGY RELIEF HOMEOPATHIC POLLINOSAN
ammi visnaga, aralia racemosa, cardiospermum halicacabum, galphimia glauca, luffa operculata, okoubaka aubrevillei, paloondo tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Bioforce AG (480344100) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bioforce AG | 480344100 | manufacture(53758-420) | |