PRENATAL, DHA- thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, ascorbic acid, niacin, folic acid, ferrous fumarate, calcium carbonate, cholecalciferol, vitamin e acetate, potassium iodide, zinc oxide, choline bitartrate, and doconexent
PureTek Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
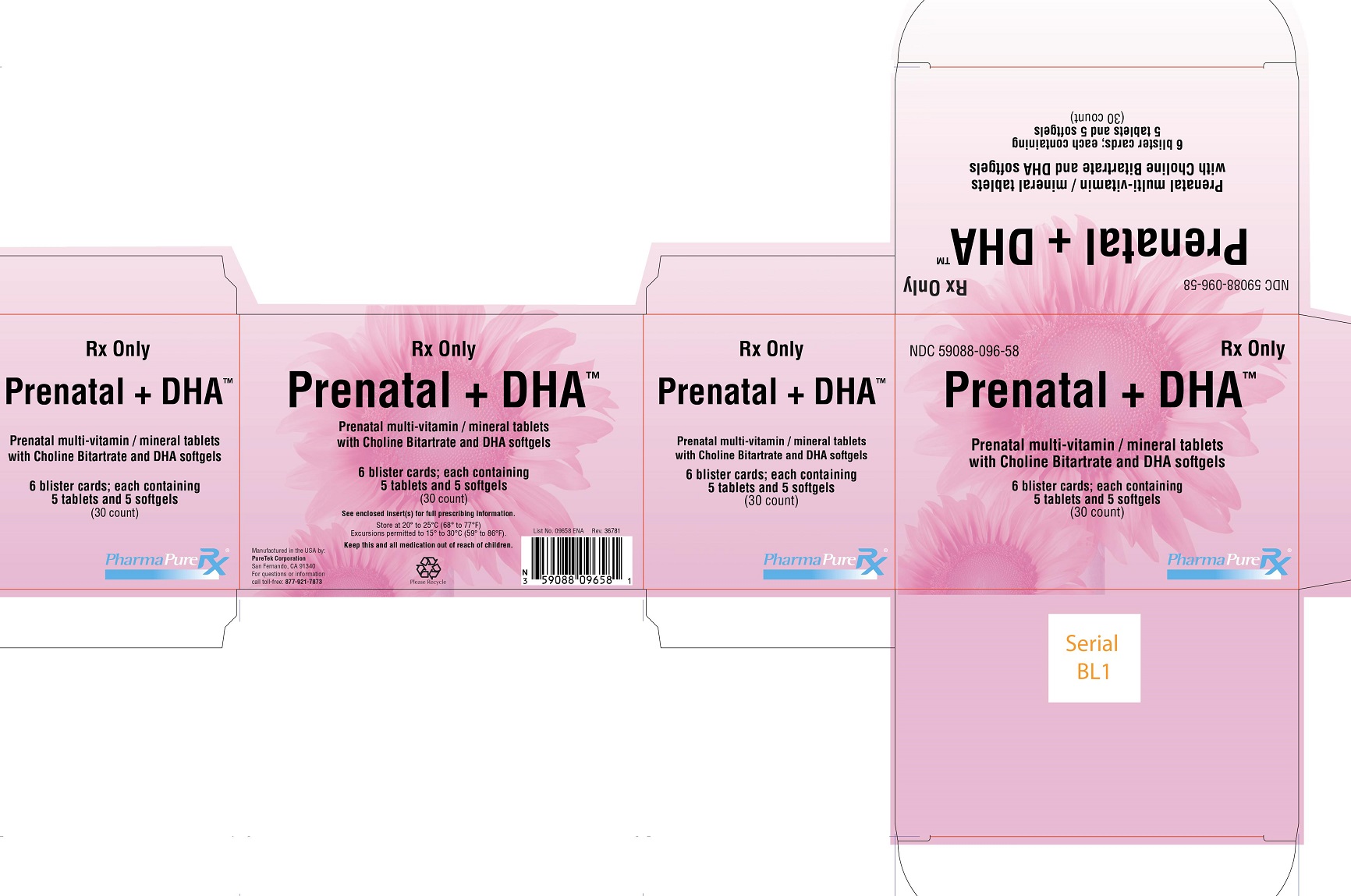
Prenatal + DHA
Prenatal multi-vitamin / mineral tablets with Choline Bitartrate and DHA softgels
Description:
Prenatal + DHA™ includes a light pink coated oblong-shaped tablet with “P658” debossed on one side of the tablet and a natural DHA softgel containing a pale orange oil.
Each tablet contains:
| Vitamin B1 (Thiamin Mononitrate) | 3 mg |
| Vitamin B2 (Riboflavin) | 3 mg |
| Vitamin B6 (Pyridoxine HCl) | 50 mg |
| Vitamin B12 (Cyanocobalamin) | 8 mcg |
| Vitamin C (Ascorbic acid) | 120 mg |
| Niacin (Niacinamide) | 20 mg |
| Folic acid | 1 mg |
| Iron (Ferrous Fumarate) | 27 mg |
| Calcium (Calcium Carbonate) | 200 mg |
| Vitamin D3 (Cholecalciferol) | 10.3 mcg |
| Vitamin E (DL-Alpha Tocopheryl Acetate) | 27 mg |
| Iodine (Potassium Iodide) | 150 mcg |
| Zinc (Zinc Oxide) | 15 mg |
| Choline Bitartrate | 55 mg |
Tablet Inactive Ingredients:
Maltodextrin, Microcrystalline Cellulose, Croscarmellose Sodium, Modified Starch, Polyvinyl Alcohol, Titanium Dioxide, Polyethylene Glycol, Citric Acid, Talc, Silica, Stearic Acid, Magnesium Stearate, Calcium Sulfate, Bovine Gelatin Hydrolyzed, Sucrose, Dicalcium Phosphate, Corn Starch, Silicon Dioxide, FD&C Red #40/Allura Red AC Aluminum Lake, Partially Hydrogenated Soybean Oil, FD&C Yellow #6/Sunset Yellow FCF Aluminum Lake, FD&C Blue #1/Brilliant Blue FCF Aluminum Lake.
Each DHA softgel contains:
|
DHA is an omega-3 fatty acid. The DHA in Prenatal + DHA™ is derived from Algal Oil (C.cohnii). 625 mg of Algal Oil is equivalent to 250 mg of DHA. Other ingredients in the DHA softgel: Gelatin, High Oleic Sunflower Oil, Glycerin, Ascorbyl Palmitate, Tocopherols, Polysorbate 80. |
|
| Docosahexaenoic Acid (DHA) from Algal Oil | 250 mg |
indications and Usage:
Prenatal + DHA™ is indicated to provide vitamin/mineral and DHA omega-3 fatty acid supplement to women throughout pregnancy, during the postnatal period for both lactating and non-lactating mothers, and throughout the childbearing years. Prenatal + DHA™ may be beneficial in improving the nutritional status of women prior to conception.
Contraindications:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Warnings:
Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential antithrombotic effects, including increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired bleeding diathesis.
Precautions:
Folic acid in doses above 0.1mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
Adverse Reactions:
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
DOSAGE AND ADMINISTRATION
One Prenatal + DHA™ tablet and one Prenatal + DHA™ softgel daily or as directed by a physician.
How Supplied
Prenatal + DHA™ is supplied as six child-resistant blister cards containing 5 Prenatal + DHA™ tablets and 5 Prenatal + DHA™ softgels each - NDC 59088-096-58.
Storage
Do not use if blister seal is broken.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Excursions permitted to 15° to 30ºC (59° to 86°F).
To report a serious adverse event or to obtain product information, contact 877-921-7873
Manufactured in the USA by:
PureTek Corporation
San Fernando, CA 91340
For questions or information
call toll-free: 877-921-7873
| PRENATAL, DHA
thiamine mononitrate, riboflavin, pyridoxine hydrochloride, cyanocobalamin, ascorbic acid, niacin, folic acid, ferrous fumarate, calcium carbonate, cholecalciferol, vitamin e acetate, potassium iodide, zinc oxide, choline bitartrate, and doconexent kit |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - PureTek Corporation (785961046) |