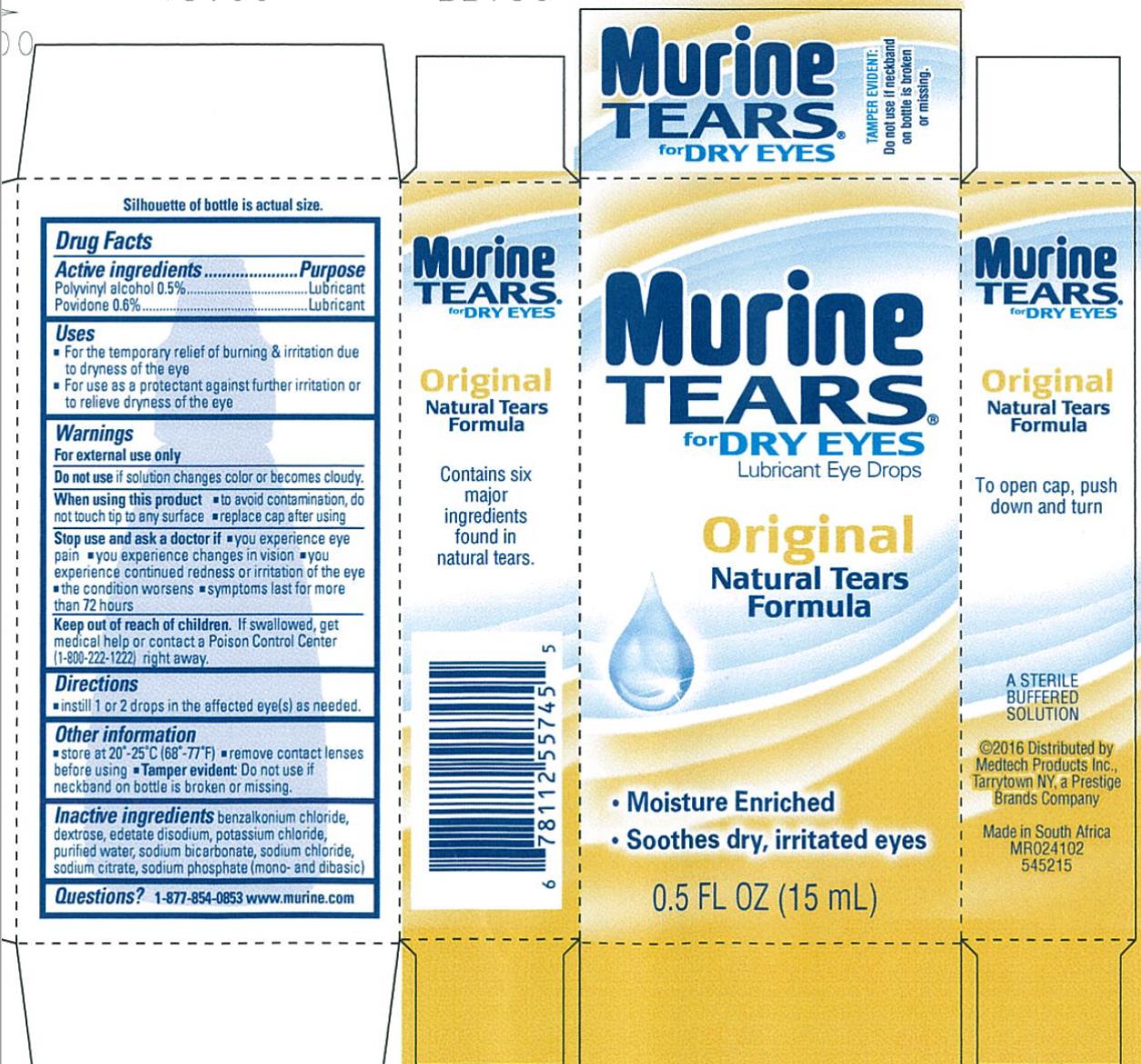
MURINE TEARS FOR DRY EYES- polyvinyl alcohol and povidone liquid
Prestige Brands Holdings, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Murine Tears for Dry Eyes
Uses
- For the temporary relief of burning & irritation due to dryness of the eye.
- For use as a protectant against further irritation or dryness of the eye.
Warnings
For external use only.
When using this product
- to avoid contamination, do not touch tip of container to any surface.
- recap cap after using.
Other information
- store at 20°- 25°C (68°-77°F)
- remove contact lenses before using
- Tamper Evident: Do not use if neckband on bottle is broken or missing.
| MURINE TEARS FOR DRY EYES
polyvinyl alcohol and povidone liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Prestige Brands Holdings, Inc. (159655021) |
Revised: 3/2023
Document Id: 50c43449-49b4-4caa-ba87-7469a363d38a
Set id: e81bb895-0743-460a-88a0-bf57ec729422
Version: 4
Effective Time: 20230306
Prestige Brands Holdings, Inc.