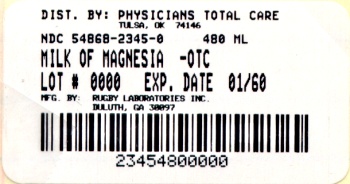
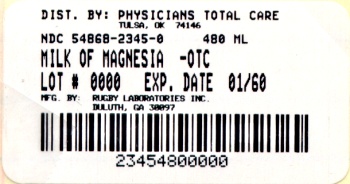
Label: MILK OF MAGNESIA- magnesium hydroxide suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-2345-0, 54868-2345-2 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0536-2470
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 24, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Ask a doctor or pharmacist before use if you are
- Stop use and ask a doctor if
- If pregnant or breast-feeding
- Keep this and all drugs out of the reach of children
-
Directions
- shake well before use
- do not exceed the maximum recommended daily dose in a 24 hour period
- dose may be taken once a day, in divided doses, or as directed by a doctor
- drink a full glass (8oz) of liquid with each dose
adults and children 12 years and older
2 to 4 tabletspoonfuls (TBSP)
children 6 to 11 years
1 to 2 tablespoonfuls (TBSP)
children under 6 years
ask a doctor
- Other information
- Inactive Ingredients
-
Principal Display Panel
Compare to
Active Ingredient in
Phillips Milk of Magnesia
Rugby Milk of Magnesia is distributed by
Rugby Laboratories, Inc.
Duluth, GA 30097
Additional barcode labeling by;
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
Milk of Magnesia
Magnesium Hydroxide 1200mg
Saline Laxative
Mint Flavor
Net Contents
16 fl oz (473 mL)
-
INGREDIENTS AND APPEARANCE
MILK OF MAGNESIA
magnesium hydroxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54868-2345(NDC:0536-2470) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM HYDROXIDE - UNII:NBZ3QY004S) MAGNESIUM HYDROXIDE 1200 mg in 15 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) SODIUM HYPOCHLORITE (UNII: DY38VHM5OD) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Product Characteristics Color Score Shape Size Flavor MINT (mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-2345-0 473 mL in 1 BOTTLE, PLASTIC 2 NDC:54868-2345-2 355 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part334 06/19/1997 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel