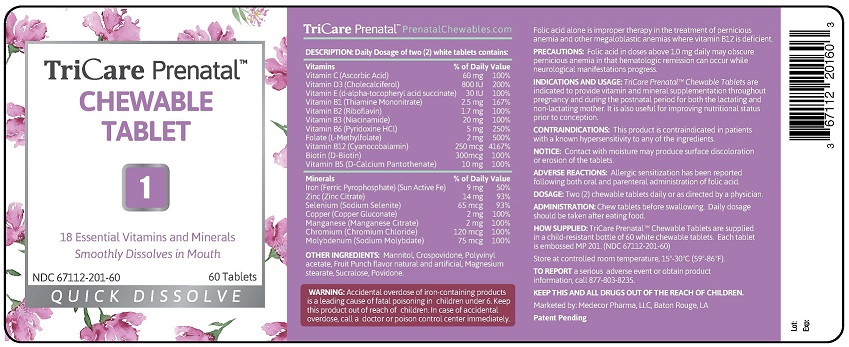
TRICARE PRENATAL QUICK DISSOLVE- 18 essential vitamins and minerals tablet, chewable
Medecor Pharma, LLC
----------
TRICARE PRENATAL CHEWABLE TABLET "1"
18 ESSENTIAL VITAMINS AND MINERALS
SMOOTHLY DISSOLVES IN MOUTH
60 TABLETS
| TRICARE PRENATAL
QUICK DISSOLVE
18 essential vitamins and minerals tablet, chewable |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| shape | ||
| size (solid drugs) | 9 mm | |
| scoring | 1 | |
| Labeler - Medecor Pharma, LLC (830621046) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sterling Pharmaceutical Services, LLC | 079569189 | manufacture | |
Revised: 12/2022
Document Id: e760f383-540b-4588-ac36-653c67343763
Set id: e7d1e359-c218-449c-a93c-a95536375273
Version: 8
Effective Time: 20221227
Medecor Pharma, LLC