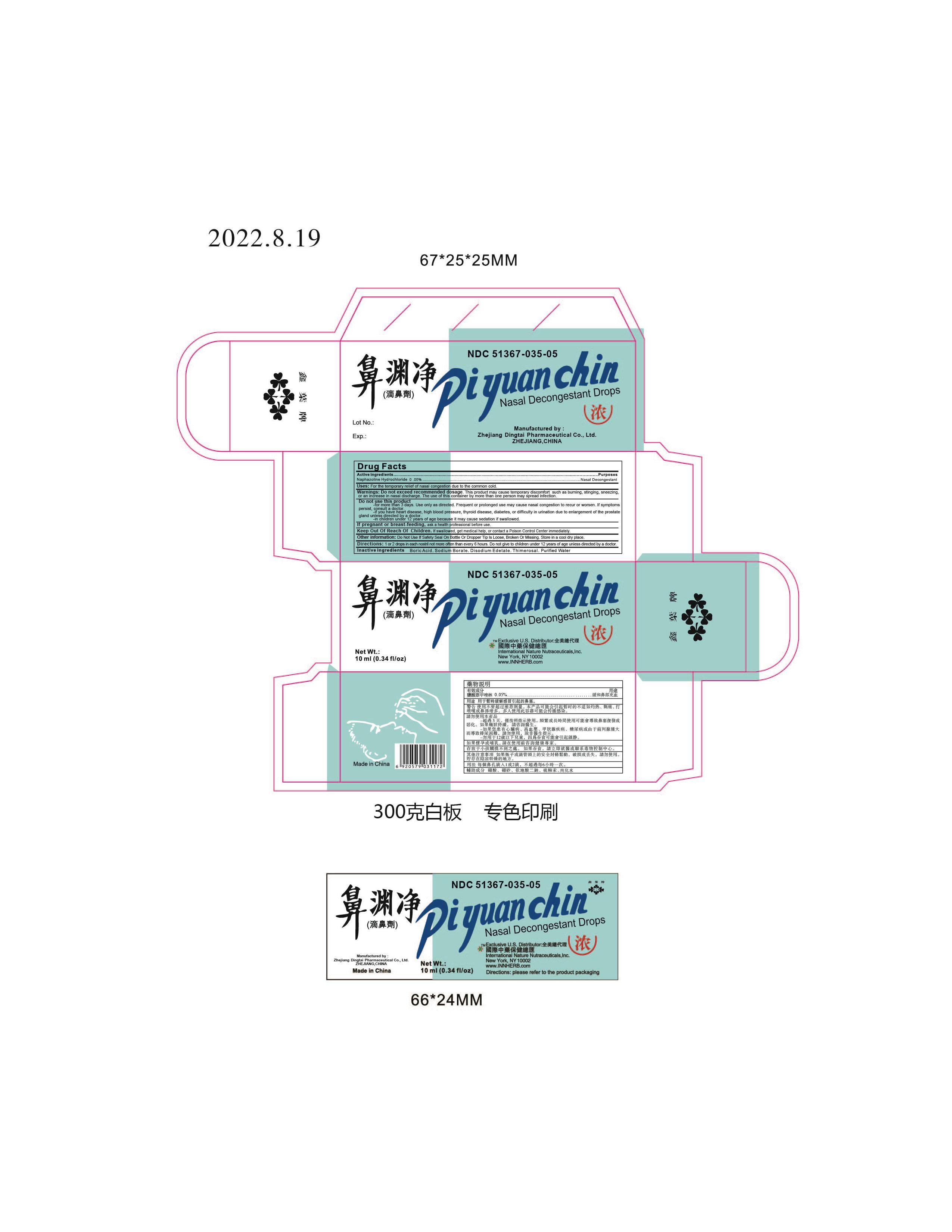
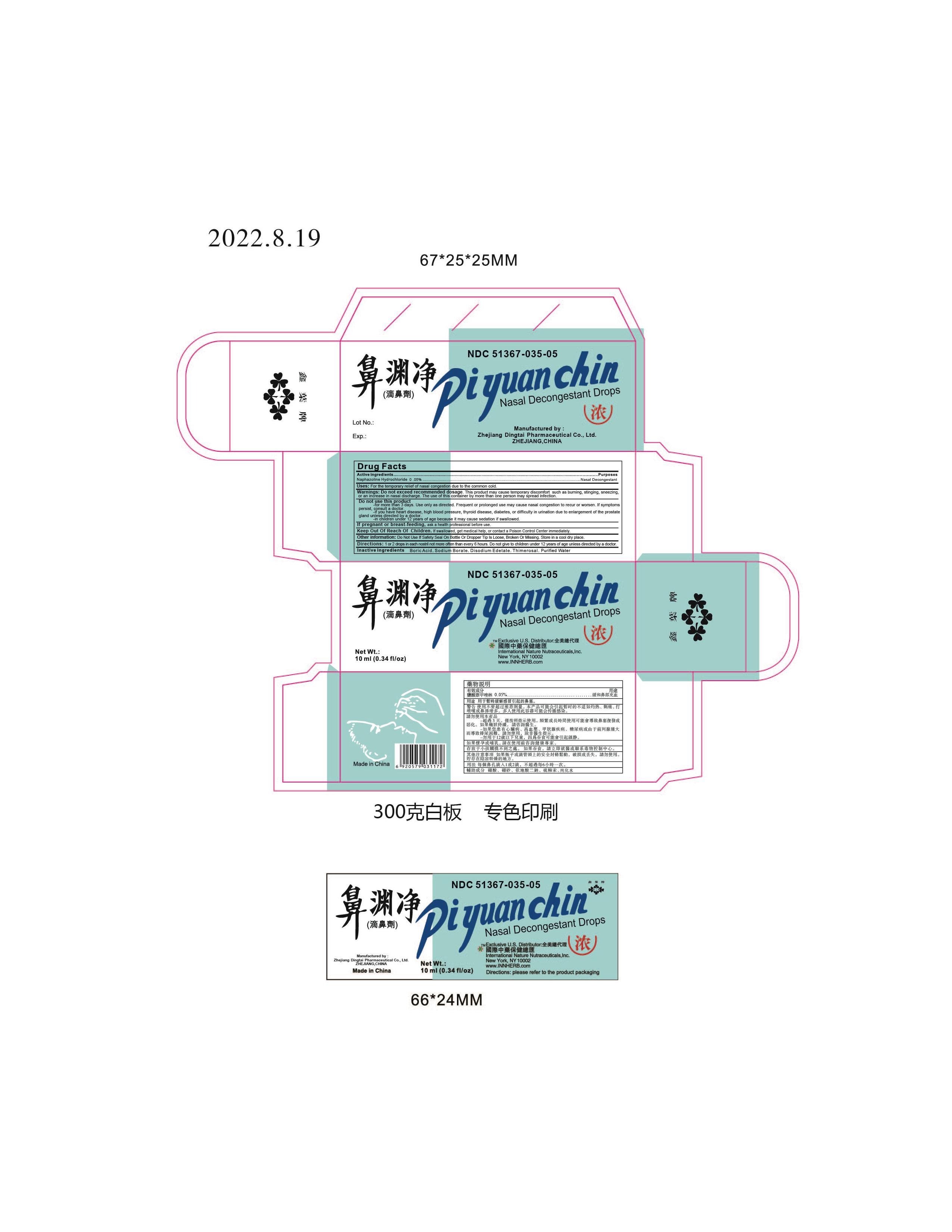
Label: PI YUAN CHIN- naphazoline hydrochloride solution/ drops
- NDC Code(s): 51367-035-05
- Packager: International Nature Nutraceuticals
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 20, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Uses:
-
Warnings: Do not exceed recommended dosage.
This product may cause temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge.
The use of this container by more than one person may spread infection.
Do not use this product
- for more than 3 days. Use only as directed. Frequent or prolonged use may cuase nasal congestion to recur or worsen. If symptons persist, consult a doctor.
- If you have heart disease, high blood pressure, thyroid disease, diabetes, or difficulty in urination due to enlargement of the prostate gland unless directed by a doctor.
- in children under 12 years of age because it may cause sedation if swallowed.
- Other information:
- Directions:
- Inactive Ingredients:
- Packaging
-
INGREDIENTS AND APPEARANCE
PI YUAN CHIN
naphazoline hydrochloride solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51367-035 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPHAZOLINE HYDROCHLORIDE (UNII: MZ1131787D) (NAPHAZOLINE - UNII:H231GF11BV) NAPHAZOLINE HYDROCHLORIDE 1 mg Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) EDETATE DISODIUM (UNII: 7FLD91C86K) THIMEROSAL (UNII: 2225PI3MOV) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51367-035-05 1 in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 07/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 07/15/2022 Labeler - International Nature Nutraceuticals (006106879) Establishment Name Address ID/FEI Business Operations Zhejiang Dingtai Pharmaceutical Co., Ltd 420598724 manufacture(51367-035)