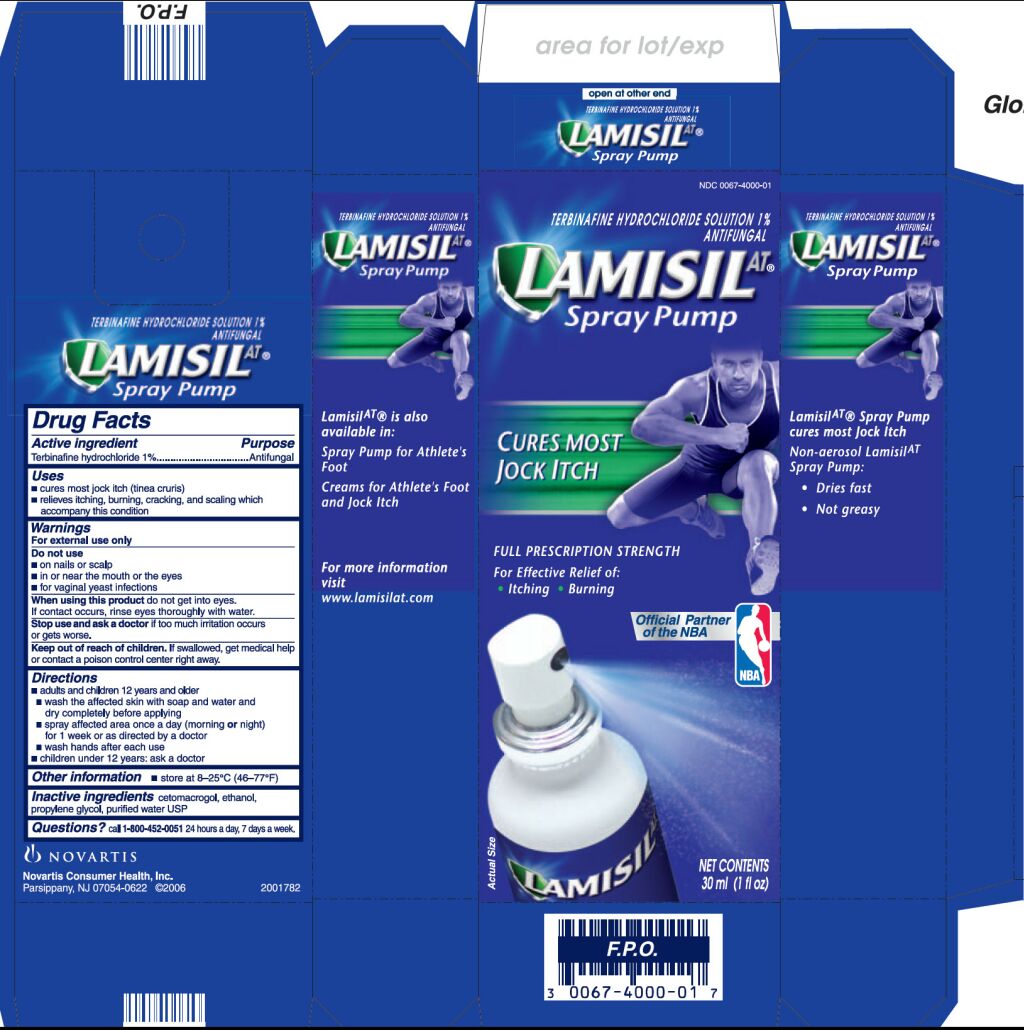
LAMISIL AT- terbinafine hydrochloride spray
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
----------
Drug Facts
Uses
- •
- cures most jock itch (tinea cruris)
- •
- relieves itching, burning, cracking, and scaling which accompany this condition
Keep Out of Reach of Children
If swallowed, get medical help or contact a poison control
center right away.
Directions
- •
- adults and children 12 years and over
- •
- wash the affected area with soap and water and dry completely before applying
- •
- spray affected area once a day (morning or night) for 1 week or as directed by a doctor
- •
- wash hands after each use
- •
- children under 12 years: ask a doctor
| LAMISIL AT
terbinafine hydrochloride spray |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |
Revised: 12/2018
Document Id: 057be0c8-6f3f-477e-a765-201eb6c4c5dd
Set id: e74fbb63-b66b-4f2e-ab77-f3916d73540f
Version: 3
Effective Time: 20181220
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC