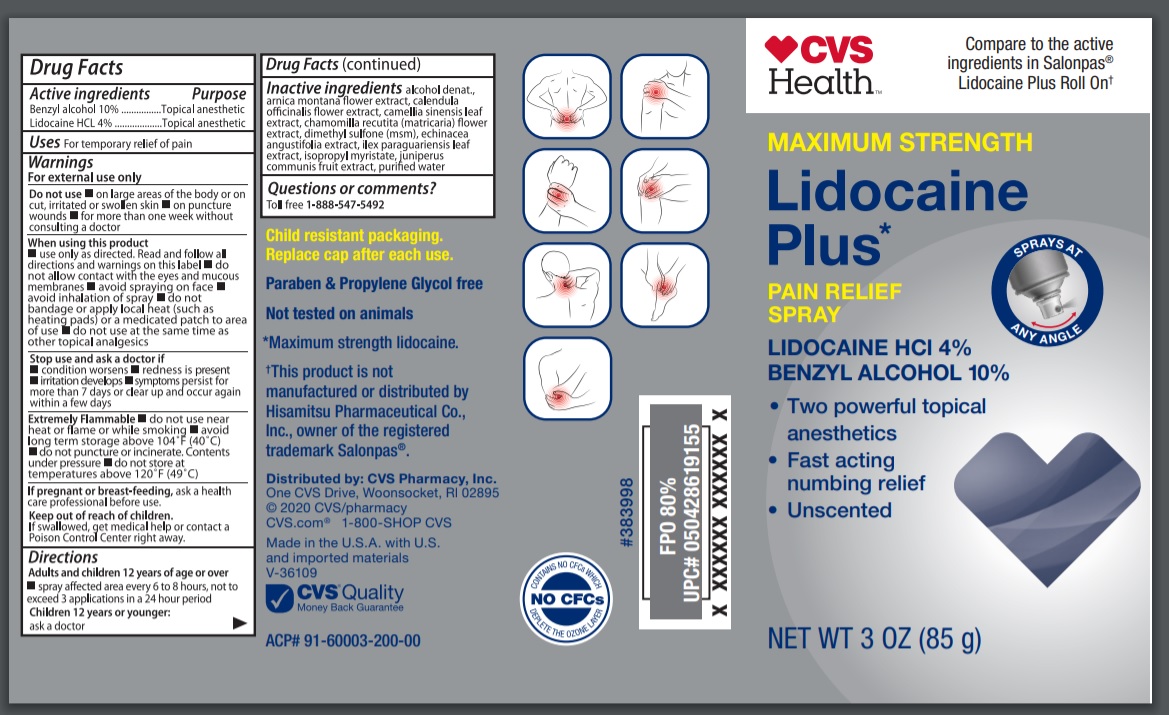
Label: CVS MAXIMUM STRENGTH LIDOCAINE PLUS- benzyl alcohol, lidocaine hydrochloride spray
- NDC Code(s): 69842-573-63
- Packager: CVS Pharmacy Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Flammable:
Keep away from fire or flame
-Do not use near heat or flame or while smoking.
-avoid long term storage above 40 degree Celcius.
-do not puncture or incinerate. COntents under pressure
-do not store at temperatures above 49 degree Celcius
Do not use
- on large areas of the body or cut or wounds or damaged skin
- on puncture wounds
- for more than one week without consulting a doctor
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- avoid spraying on face
- avoid inhalation of spray
- do not bandage or apply local heat such as heating pads or a medicated patch to area of use
- do not use at the same time as other topical analgesics
- Directions
- Other information
-
Inactive ingredients
alcohol denatured, arnica montana flower extract, calendula officinalis flower extract, camellia sinensis leaf extract, chamomilla recutita (matricaria) flower extract , dimethyl sulfone (msm), Echinacea angustifolia extract, ilex paraguariensis leaf extract, isopropyl myristate, juniperus communis fruit extract, purified water
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CVS MAXIMUM STRENGTH LIDOCAINE PLUS
benzyl alcohol, lidocaine hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-573 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZYL ALCOHOL (UNII: LKG8494WBH) (BENZYL ALCOHOL - UNII:LKG8494WBH) BENZYL ALCOHOL 10 g in 100 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 g in 100 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CHAMOMILE (UNII: FGL3685T2X) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) ECHINACEA ANGUSTIFOLIA WHOLE (UNII: VB06AV5US8) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) JUNIPER BERRY (UNII: O84B5194RL) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-573-63 1 in 1 BOX 05/15/2020 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/15/2020 Labeler - CVS Pharmacy Inc. (062312574)