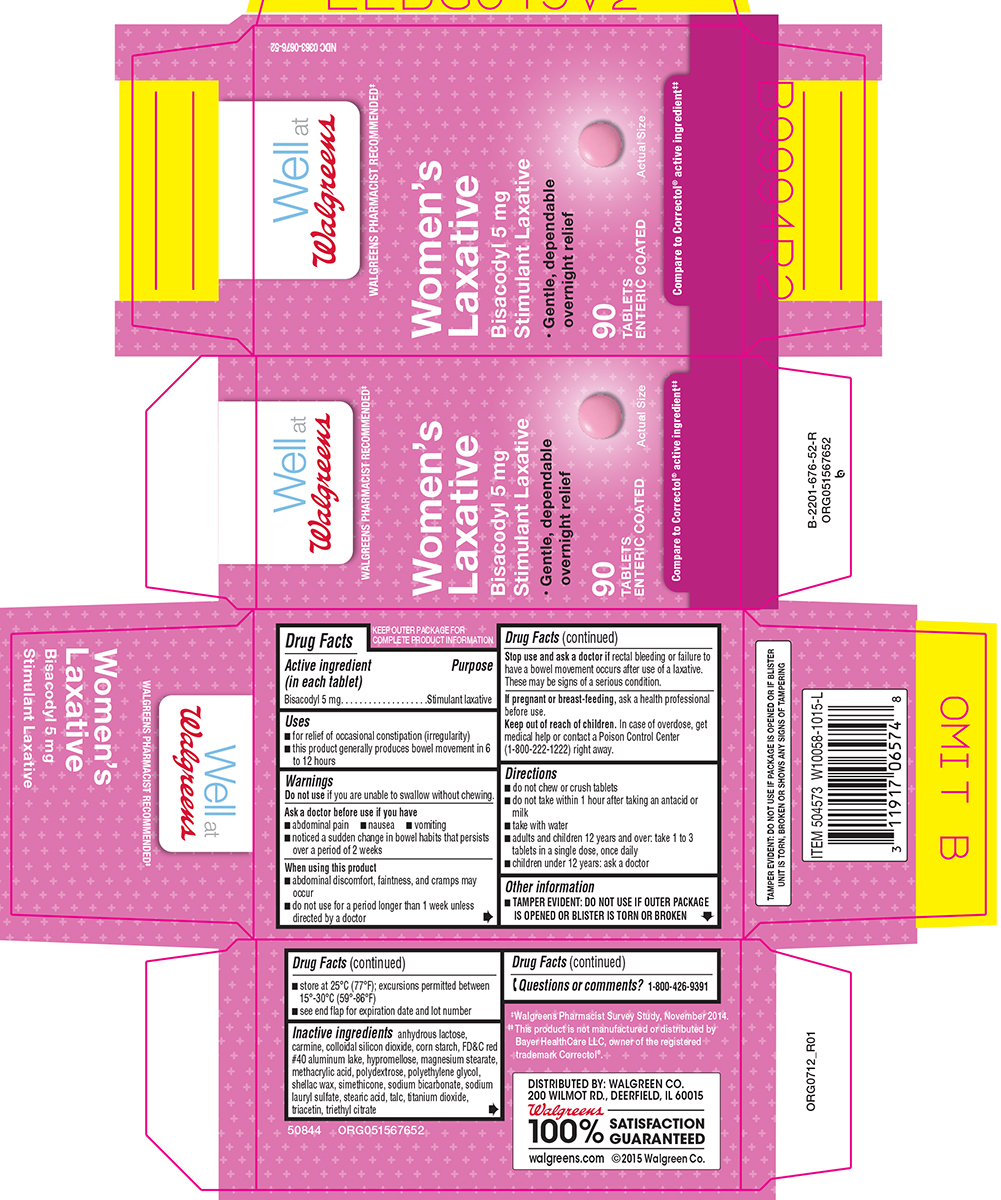
WOMENS LAXATIVE- bisacodyl tablet, coated
Walgreen Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Walgreens 44-676
Uses
- for relief of occasional constipation (irregularity)
- this product generally produces bowel movement in 6 to 12 hours
Warnings
Ask a doctor before use if you have
- abdominal pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
When using this product
- abdominal discomfort, faintness, and cramps may occur
- do not use for a period longer than 1 week unless directed by a doctor
Directions
- do not chew or crush tablets
- do not take within 1 hour after taking an antacid or milk
- take with water
- adults and children 12 years and over: take 1 to 3 tablets in a single dose, once daily
- children under 12 years: ask a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
anhydrous lactose, carmine, colloidal silicon dioxide, corn starch, FD&C red #40 aluminum lake, hypromellose, magnesium stearate, methacrylic acid, polydextrose, polyethylene glycol, shellac wax, simethicone, sodium bicarbonate, sodium lauryl sulfate, stearic acid, talc, titanium dioxide, triacetin, triethyl citrate
Principal display panel
Well at
Walgreens
WALGREENS PHARMACIST RECOMMENDED‡
NDC 0363-0676-52
Women's
Laxative
Bisacodyl 5 mg
Stimulant Laxative
•Gentle, dependable
overnight relief
90
TABLETS
ENTERIC COATED
Compare to Correctol® active ingredient‡‡
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015
Walgreens
100% SATISFACTION GUARANTEED
walgreens.com ©2015 Walgreen Co.
‡Walgreens Pharmacist Survey Study, November 2014.
‡‡This product is not manufactured or distributed by Bayer HealthCare LLC, owner of the registered trademark Correctol®.
50844 ORG051567652
Walgreens 44-676
| WOMENS LAXATIVE
bisacodyl tablet, coated |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Walgreen Company (008965063) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(0363-0676) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(0363-0676) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | PACK(0363-0676) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | PACK(0363-0676) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | PACK(0363-0676) | |