Label: METAXALONE tablet
- NDC Code(s): 55700-911-30, 55700-911-90
- Packager: Quality Care Products, LLC
- This is a repackaged label.
- Source NDC Code(s): 0591-2341
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated November 18, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Metaxalone Tablets, USP are available as an 800 mg capsule-shaped, scored white to off-white tablet.
Chemically, metaxalone is 5-[(3,5-dimethylphenoxy) methyl]-2-oxazolidinone. The molecular formula is C12H15NO3, which corresponds to a molecular weight of 221.25. The structural formula is:
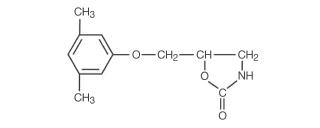
Metaxalone, USP is a white to almost white, odorless crystalline powder freely soluble in chloroform, soluble in methanol and in 96% ethanol, but practically insoluble in ether or water.
Each tablet contains 800 mg metaxalone, USP and the following inactive ingredients: alginic acid, ammonium alginate, calcium alginate, corn starch, magnesium stearate and pregelatinized starch (starch 1500 partially pregelatinized maize starch).
USP Dissolution Test Pending.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of action of metaxalone in humans has not been established, but may be due to general central nervous system (CNS) depression. Metaxalone has no direct action on the contractile mechanism of striated muscle, the motor end plate, or the nerve fiber.
Pharmacokinetics
The pharmacokinetics of metaxalone have been evaluated in healthy adult volunteers after single dose administration of metaxalone under fasted and fed conditions at doses ranging from 400 mg to 800 mg.
Absorption
Peak plasma concentrations of metaxalone occur approximately 3 hours after a 400 mg oral dose under fasted conditions. Thereafter, metaxalone concentrations decline log-linearly with a terminal half-life of 9.0 ± 4.8 hours. Doubling the dose of metaxalone from 400 mg to 800 mg results in a roughly proportional increase in metaxalone exposure as indicated by peak plasma concentrations (Cmax) and area under the curve (AUC). Dose proportionality at doses above 800 mg has not been studied. The absolute bioavailability of metaxalone is not known.
The single-dose pharmacokinetic parameters of metaxalone in two groups of healthy volunteers are shown in Table 1.
Table 1: Mean (%CV) Metaxalone Pharmacokinetic Parameters Dose
(mg)Cmax
(ng/mL)Tmax
(h)AUC∞
(ng•h/mL)t½
(h)CL/F
(L/h)4001 983 (53) 3.3 (35) 7479 (51) 9.0 (53) 68 (50) 8002 1816 (43) 3.0 (39) 15044 (46) 8.0 (58) 66 (51) 1Subjects received 1x400 mg tablet under fasted conditions (N=42)
2Subjects received 2x400 mg tablets under fasted conditions (N=59)Food Effects
A randomized, two-way, crossover study was conducted in 42 healthy volunteers (31 males, 11 females) administered one 400 mg metaxalone tablet under fasted conditions and following a standard high-fat breakfast. Subjects ranged in age from 18 to 48 years (mean age = 23.5 ± 5.7 years). Compared to fasted conditions, the presence of a high fat meal at the time of drug administration increased Cmax by 177.5% and increased AUC (AUC0-t, AUC∞) by 123.5% and 115.4%, respectively. Time-to-peak concentration (Tmax) was also delayed (4.3 h versus 3.3 h) and terminal half-life was decreased (2.4 h versus 9.0 h) under fed conditions compared to fasted.
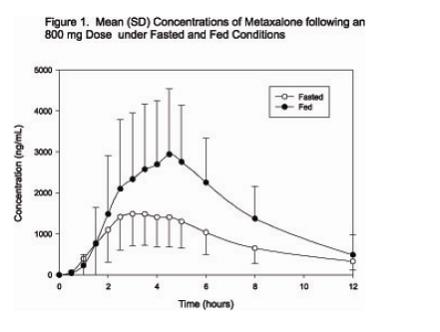
In a second food effect study of similar design, two 400 mg metaxalone tablets (800 mg) were administered to healthy volunteers (N=59, 37 males, 22 females), ranging in age from 18 to 50 years (mean age = 25.6± 8.7 years). Compared to fasted conditions, the presence of a high fat meal at the time of drug administration increased Cmax by 193.6% and increased AUC (AUC0-t, AUC∞) by 146.4% and 142.2%, respectively. Time-to-peak concentration (Tmax) was also delayed (4.9 h versus 3.0 h) and terminal half-life was decreased (4.2 h versus 8.0 h) under fed conditions compared to fasted conditions. Similar food effect results were observed in the above study when one metaxalone 800 mg tablet was administered in place of two metaxalone 400 mg tablets. The increase in metaxalone exposure coinciding with a reduction in half-life may be attributed to more complete absorption of metaxalone in the presence of a high fat meal (Figure 1).
Distribution, Metabolism, and Excretion
Although plasma protein binding and absolute bioavailability of metaxalone are not known, the apparent volume of distribution (V/F ~ 800 L) and lipophilicity (log P = 2.42) of metaxalone suggest that the drug is extensively distributed in the tissues. Metaxalone is metabolized by the liver and excreted in the urine as unidentified metabolites. Hepatic Cytochrome P450 enzymes play a role in the metabolism of metaxalone. Specifically, CYP1A2, CYP2D6, CYP2E1, and CYP3A4 and, to a lesser extent, CYP2C8, CYP2C9, and CYP2C19 appear to metabolize metaxalone.
Metaxalone does not significantly inhibit major CYP enzymes such as CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4. Metaxalone does not significantly induce major CYP enzymes such as CYP1A2, CYP2B6, and CYP3A4 in vitro.
Pharmacokinetics in Special Populations
Age:
The effects of age on the pharmacokinetics of metaxalone were determined following single administration of two 400 mg tablets (800 mg) under fasted and fed conditions. The results were analyzed separately, as well as in combination with the results from three other studies. Using the combined data, the results indicate that the pharmacokinetics of metaxalone are significantly more affected by age under fasted conditions than under fed conditions, with bioavailability under fasted conditions increasing with age.
The bioavailability of metaxalone under fasted and fed conditions in three groups of healthy volunteers of varying age is shown in Table 2.
Table 2: Mean (%CV) Pharmacokinetic Parameters Following Single Administration of Two 400 mg Metaxalone Tablets (800 mg) under Fasted and Fed Conditions Younger Volunteers Older Volunteers Age (years) 25.6 ± 8.7 39.3 ± 10.8 71.5 ± 5.0 N 59 21 23 Food Fasted Fed Fasted Fed Fasted Fed Cmax
(ng/mL)1816
(43)3510
(41)2719
(46)2915
(55)3168
(43)3680
(59)Tmax(h)
3.0
(39)4.9
(48)3.0
(40)8.7
(91)2.6
(30)6.5
(67)AUC0-t
(ng·h/mL)14531
(47)20683
(41)19836
(40)20482
(37)23797
(45)24340
(48)AUC∞
(ng·h/mL)15045
(46)20833
(41)20490
(39)20815
(37)24194
(44)24704
(47)Gender:
The effect of gender on the pharmacokinetics of metaxalone was assessed in an open label study, in which 48 healthy adult volunteers (24 males, 24 females) were administered two metaxalone 400 mg tablets (800 mg) under fasted conditions. The bioavailability of metaxalone was significantly higher in females compared to males as evidenced by Cmax (2115 ng/mL versus 1335 ng/mL) and AUC∞ (17884 ng·h/mL versus 10328 ng·h/mL). The mean half-life was 11.1 hours in females and 7.6 hours in males. The apparent volume of distribution of metaxalone was approximately 22% higher in males than in females, but not significantly different when adjusted for body weight. Similar findings were also seen when the previously described combined dataset was used in the analysis.
-
INDICATIONS AND USAGE
Metaxalone tablets are indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomforts associated with acute, painful musculoskeletal conditions. The mode of action of this drug has not been clearly identified, but may be related to its sedative properties. Metaxalone does not directly relax tense skeletal muscles in man.
- CONTRAINDICATIONS
-
WARNINGS
Serotonin Syndrome
Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of serotonergic drugs with metaxalone used within the recommended dosage range (see PRECAUTIONS: Drug Interactions) and with metaxalone as a single agent taken at doses higher than the recommended dose (see OVERDOSAGE). Serotonergic drugs include selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, opioids (particularly fentanyl, meperidine, and methadone), drugs that affect the serotonergic neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), and drugs that impair metabolism of serotonin (including monoamine oxidase (MAO) inhibitors, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue) (see PRECAUTIONS: Drug Interactions).
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination, rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms generally occurs within several hours to a few days, but may occur later than that. Discontinue metaxalone if serotonin syndrome is suspected.
Risks from Concomitant Use with Alcohol or other CNS Depressants
The sedative effects of metaxalone and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants (TCAs)) may be additive. Exercise caution with patients who take more than one of these CNS depressants simultaneously. Follow patients closely for signs and symptoms of respiratory depression and sedation (see PRECAUTIONS: Drug Interactions).
-
PRECAUTIONS
Metaxalone should be administered with great care to patients with pre-existing liver damage. Serial liver function studies should be performed in these patients.
False-positive Benedict’s tests, due to an unknown reducing substance, have been noted. A glucose-specific test will differentiate findings.
Taking metaxalone with food may enhance general CNS depression; elderly patients may be especially susceptible to this CNS effect (see CLINICAL PHARMACOLOGY: Pharmacokinetics andPRECAUTIONS: Information for Patients).
Information for Patients
Driving or Operating Heavy Machinery
Metaxalone may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle, especially when used with alcohol or other CNS depressants.
Serotonin Syndrome
Inform patients that metaxalone tablets could cause a rare but potentially life-threatening condition resulting from administration of doses higher than the recommended dose or from concomitant administration of serotonergic drugs with metaxalone tablets used within the recommended dosage range. Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop. Instruct patients to inform their healthcare providers if they are taking, or plan to take, serotonergic medications (see WARNINGS, PRECAUTIONS: Drug Interactions, and OVERDOSAGE).
Drug Interactions
CNS Depressants
The sedative effects of metaxalone and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants (TCAs)) may be additive. Exercise caution with patients who take more than one of these CNS depressants simultaneously. Follow patients closely for signs and symptoms of respiratory depression and sedation (see WARNINGS).
Serotonergic Drugs
Serotonin syndrome has resulted from concomitant use of serotonergic drugs with metaxalone used within the recommended dosage range (see WARNINGS). If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue metaxalone if serotonin syndrome is suspected.
Examples of serotonergic drugs include: selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, opioids (particularly fentanyl, meperidine, and methadone), drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of metaxalone has not been determined.
Pregnancy
Reproduction studies in rats have not revealed evidence of impaired fertility or harm to the fetus due to metaxalone. Post marketing experience has not revealed evidence of fetal injury, but such experience cannot exclude the possibility of infrequent or subtle damage to the human fetus. Safe use of metaxalone has not been established with regard to possible adverse effects upon fetal development. Therefore, metaxalone tablets should not be used in women who are or may become pregnant and particularly during early pregnancy unless, in the judgement of the physician, the potential benefits outweigh the possible hazards.
-
ADVERSE REACTIONS
The most frequent reactions to metaxalone include:
CNS: drowsiness, dizziness, headache, and nervousness or “irritability”;
Digestive: nausea, vomiting, gastrointestinal upset.
Other adverse reactions are:
Immune System: anaphylaxis, hypersensitivity reaction, rash with or without pruritus;
Hematologic: leukopenia; hemolytic anemia;
Hepatobiliary: jaundice;
CNS: cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of serotonergic drugs with metaxalone used within the recommended dosage range and with metaxalone as a single agent taken at doses higher than the recommended dose (see WARNINGS, PRECAUTIONS: Drug Interactions, and OVERDOSAGE).
To report SUSPECTED ADVERSE REACTIONS, contact Actavis at 1-888-838-2872 or FDA at 1-800-FDA-1088 www.fda.gov/medwatch.
-
OVERDOSAGE
Deaths by deliberate or accidental overdose have occurred with metaxalone, particularly in combination with antidepressants, and have been reported with this class of drug in combination with alcohol.
Serotonin syndrome has been reported when metaxalone was used at doses higher than the recommended dose (see WARNINGS and ADVERSE REACTIONS).
When determining the LD50 in rats and mice, progressive sedation, hypnosis, and finally respiratory failure were noted as the dosage increased. In dogs, no LD50 could be determined as the higher doses produced an emetic action in 15 to 30 minutes.
Treatment - Gastric lavage and supportive therapy. Consultation with a regional poison control center is recommended.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Metaxalone Tablets, USP are available as an 800 mg capsule-shaped, scored white to off-white tablet, inscribed with “31 90” on the scored side and “WPI” on the other side. Metaxalone tablets USP, 800 mg has functional scoring.
55700-911-90
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Manufactured by:
Actavis Laboratories FL, Inc.
Fort Lauderdale, FL 33314 USADistributed by:
Actavis Pharma, Inc.
Parsippany, NJ 07054 USARev. A 5/2020
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METAXALONE
metaxalone tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55700-911(NDC:0591-2341) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METAXALONE (UNII: 1NMA9J598Y) (METAXALONE - UNII:1NMA9J598Y) METAXALONE 800 mg Inactive Ingredients Ingredient Name Strength ALGINIC ACID (UNII: 8C3Z4148WZ) AMMONIUM ALGINATE (UNII: Q9QKJ39Q3X) CALCIUM ALGINATE (UNII: 8P20S56HZI) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (white to off-white) Score 2 pieces Shape OVAL (capsule-shaped) Size 19mm Flavor Imprint Code 31;90;WPI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55700-911-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/17/2021 2 NDC:55700-911-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 08/17/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203695 08/17/2021 Labeler - Quality Care Products, LLC (831276758) Establishment Name Address ID/FEI Business Operations Quality Care Products, LLC 831276758 repack(55700-911)


