Label: PRENATE ESSENTIAL- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, pyridoxine hydrochloride, folic acid, 5-methyltetrahydrofolic acid, cyanocobalamin, biotin, calcium formate, ferrous asparto glycinate, potassium iodide, magnesium oxide, doconexent and icosapent capsule, gelatin coated
- NDC Code(s): 75854-313-30
- Packager: Avion Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
-
INDICATIONS & USAGE
INDICATIONS: PRENATE ESSENTIAL ® is a multivitamin/multimineral fatty acid dietary supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and nonlactating mothers.
PRENATE ESSENTIAL ® can also be beneficial in improving the nutritional status of women prior to conception
- CONTRAINDICATIONS
-
WARNINGS
WARNING: Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and International Normalized Ratio (INR). Administration of omega-3 fatty acids should be avoided in patients taking anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
-
PRECAUTIONS
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B 12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
- BOXED WARNING (What is this?)
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
-
HOW SUPPLIED
HOW SUPPLIED: Bottles of 30 softgels (75854-313-30).
The listed product number is not a National Drug Code. Instead, Avion Pharmaceuticals has assigned a product code formatted according to standard industry practice to meet the formatting requirements of pharmacy and healthcare insurance computer systems.
- STORAGE AND HANDLING
-
SPL UNCLASSIFIED SECTION
MANUFACTURED FOR: Avion Pharmaceuticals, LLC
Atlanta, GA 30005
1-888-61-AVION
Rev. 0519-01
Formical ® is a registered trademark of Nephro-Tech 1, LLC, covered by one or more claims of U.S. Patent No. 6,528,542.
Sumalate ® is a registered trademark of Albion Laboratories, Inc., covered by one or more claims of U.S. Patent Nos. 5,516,925, 6,716,814, 8,007,846, and 8,425,956.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
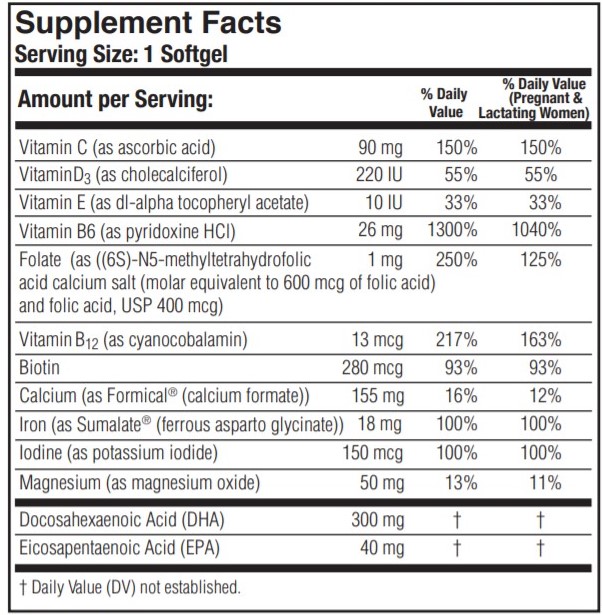
PRENATE ESSENTIAL
ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, pyridoxine hydrochloride, folic acid, 5-methyltetrahydrofolic acid, cyanocobalamin, biotin, calcium formate, ferrous asparto glycinate, potassium iodide, magnesium oxide, doconexent and icosapent capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75854-313 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ICOSAPENT (UNII: AAN7QOV9EA) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT 40 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 90 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 220 [iU] .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 10 [iU] PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 26 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 400 ug 5-METHYLTETRAHYDROFOLIC ACID (UNII: TYK22LML8F) (5-METHYLTETRAHYDROFOLIC ACID - UNII:TYK22LML8F) 5-METHYLTETRAHYDROFOLIC ACID 600 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 13 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 280 ug CALCIUM FORMATE (UNII: NP3JD65NPY) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 155 mg FERROUS ASPARTO GLYCINATE (UNII: H7426RGB3L) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 18 mg POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) IODIDE ION 150 ug MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CATION 50 mg DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 300 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) WHITE WAX (UNII: 7G1J5DA97F) WATER (UNII: 059QF0KO0R) SOYBEAN OIL (UNII: 241ATL177A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color green, yellow (opaque) Score no score Shape OVAL Size 24mm Flavor Imprint Code Prenate Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75854-313-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/18/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/18/2014 Labeler - Avion Pharmaceuticals, LLC (040348516) Establishment Name Address ID/FEI Business Operations Avion Pharmaceuticals, LLC 040348516 manufacture(75854-313)