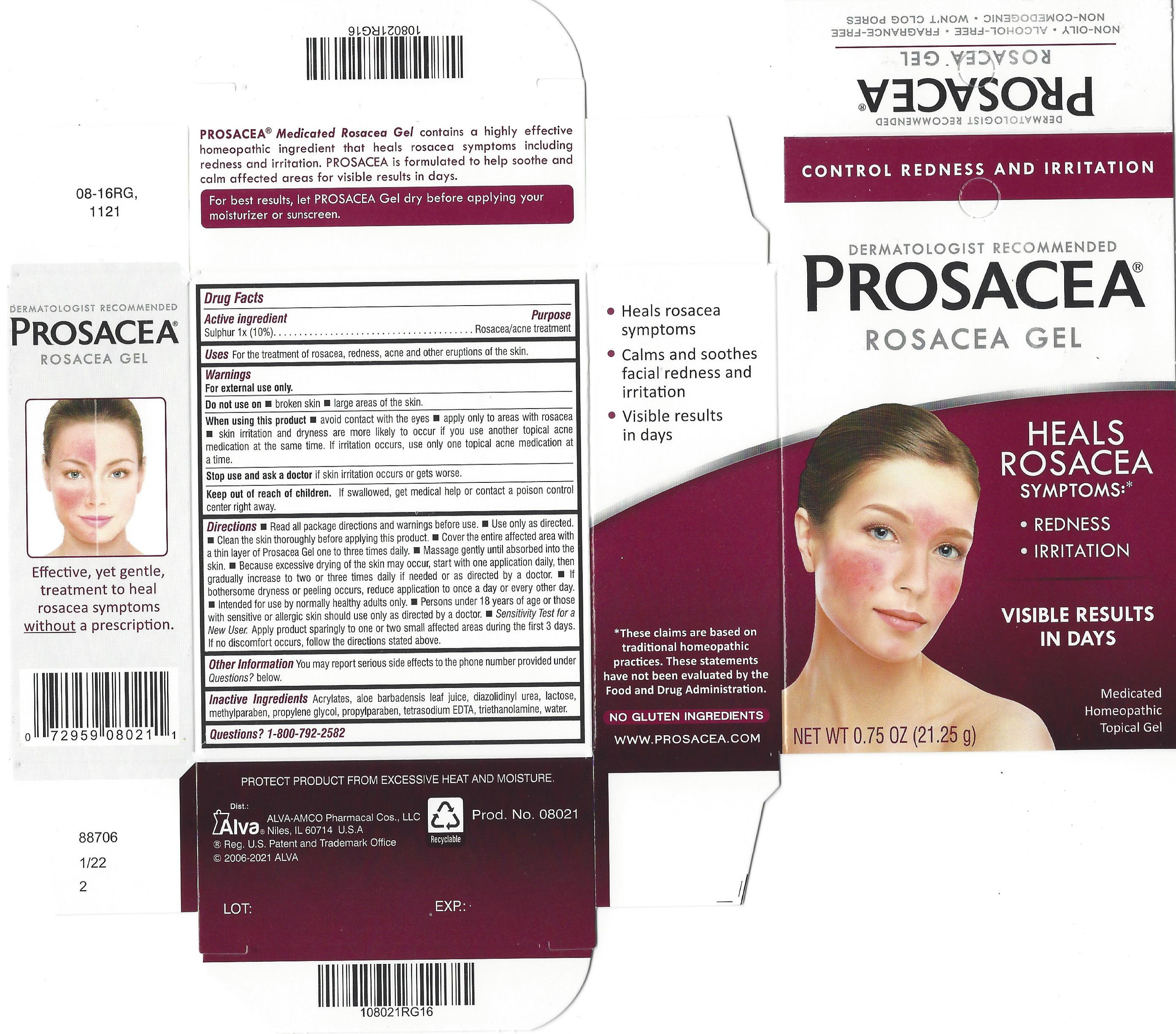
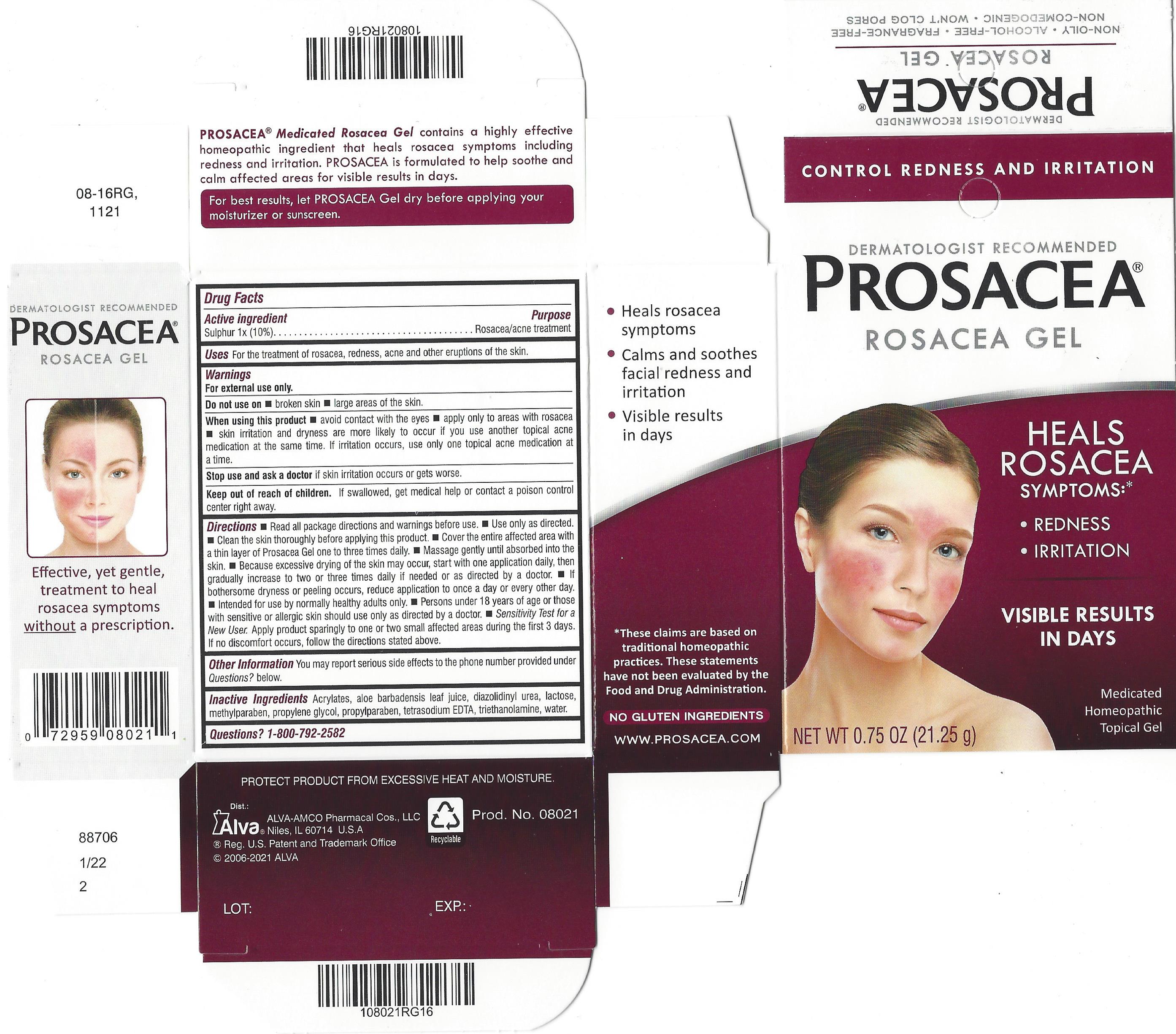
Label: PROSACEA- sulphur gel
- NDC Code(s): 52389-173-01, 52389-173-21, 52389-173-42
- Packager: Alva-Amco Pharmacal Companies, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 29, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use on
- When using this product
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Read all package directions and warnings before use.
- Use only as directed.
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer of Prosacea Gel one to three times daily.
- Massage gently until absorbed into the skin.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Intended for use by normally healthy adults only.
- Persons under 18 years of age or those with sensitive or allergic skin should use only as directed by a doctor.
- Sensitivity Test for a New User. Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
- Other information
- Inactive ingredients
- QUESTIONS
-
PATIENT PACKAGE INSERT
Dermatologist Recommended
PROSACEA ®
Medicated Rosacea Gel
Heal, Don't Conceal TM, Your Rosacea Symptoms
Control Rosacea Symptoms: Redness, Pimples, Irritation
and See Visible Results in Just Days
WHAT IS ROSACEA?
Rosacea is a condition that is characterized by persistent redness and inflammation of the skin on the cheeks, nose, chin or forehead. Rosacea is generally thought to affect those with fair skin, though it can affect all skin types. Symptoms may also include acne-like bumps and visible blood vessels. Rosacea is commonly known to be aggravated by anything that causes a flushing reaction (increased blood flow) in the skin, including alcohol consumption, exposure to sun or extreme temperatures, spicy foods or other irritants. Rosacea symptoms do not typically disappear if left untreated and in some cases can worsen. Symptoms may vary among sufferers, with intermittent episodes of symptom improvement and worsening over time. Generally, rosacea progresses through three symptomatic stages:
Stage 1: In the earliest stage of rosacea, the most common symptoms can include subtle to moderate redness, stinging, burning, dryness or tightness of skin. Tiny visible blood vessels (telangiectasia) may also appear on the skin's surface. Skin may begin to react to certain cosmetics, and may also be more reactive to temperature changes or become sensitive to certain foods.
Stage 2: As the condition progresses, redness becomes more persistent. Larger areas of the face may become affected. The formation of papules (red bumps) or pustules (resembling pimples) and swelling may develop. Facial pores and sebaceous follicles may become larger and more prominent.
Stage 3: Generally, stage-3 rosacea is less common, but individuals diagnosed with this advanced stage of rosacea exhibit all characteristics of stages 1 and 2. Facial features may become thicker, irregular or even exhibit gradual deformation. Affected areas of the skin become a deeper shade of red. The sebaceous glands may be extremely enlarged, contributing to further redness, inflammation, papules, pustules and swelling of facial features. If you believe you may have rosacea, do not delay in consulting a doctor for proper diagnosis and advice.
SUGGESTIONS FOR HOW TO AVOID AND CONTROL FLARE-UPS:
Medical experts have suggested some ways to avoid aggravating symptoms which include:
- Avoid direct sunlight as rosacea symptoms can be triggered or worsened by exposure to sunlight. When in the sun, use a mild sunscreen.
- Avoid rubbing or scrubbing affected areas, which can irritate the skin or stimulate increased blood flow to the areas.
- Use mild soaps, moisturizers and sunscreens and be sure to gently apply them to the face in order to avoid irritating affected areas.
- Avoid hot drinks, spicy foods and alcoholic beverages that can cause flushing.
- Protect your skin from extreme hot and cold conditions.
- Avoid cosmetics, soaps, and other facial products that contain ingredients such as alcohol, menthol and fragrances.
- Keep a log of any products, medicines, foods or weather and/or environmental conditions that may trigger flushing episodes or symptom flare-ups in order to avoid them in the future.
- Consult your doctor for treatment recommendations.
SOME ADDITIONAL SUGGESTIONS FOR OVERALL GOOD SKIN HEALTH:
- Get adequate rest
- Avoid emotional stress where possible
- Avoid excessive/stressful exercise which might cause skin flushing
- Drink adequate amounts of water daily
- Keep hands and face clean
- Shampoo hair regularly using a mild antibacterial shampoo
- Avoid touching affected areas
- Use a broad-spectrum, SPF 25+ sunscreen and mild moisturizer daily
HOW PROSACEA ® GEL CAN HELP
PROSACEA contains sulphur 1x, a medically recognized homeopathic ingredient that has been recognized for relief of common rosacea symptoms such as redness, bumps and irritation, when used as directed. PROSACEA's non-oily formula is non-comedogenic (doesn't cause pimples) and won't clog pores. It is important for successful results that once treatment is started with PROSACEA, it is continued uninterrupted, carefully following and adhering to package directions until satisfactory results are achieved or given further advice from a doctor.
For Best Results: After applying Prosacea Gel to your face, let it absorb fully into your skin before applying your moisturizer or sunscreen.
No Gluten Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROSACEA
sulphur gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52389-173 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 1 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE SODIUM (UNII: MP1J8420LU) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) CARBOMER COPOLYMER TYPE A (UNII: 71DD5V995L) LACTOSE (UNII: J2B2A4N98G) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52389-173-21 1 in 1 CARTON 02/06/2006 1 NDC:52389-173-01 21 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:52389-173-42 2 in 1 CARTON 02/03/2020 2 NDC:52389-173-01 21 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/06/2006 Labeler - Alva-Amco Pharmacal Companies, Inc. (042074856)