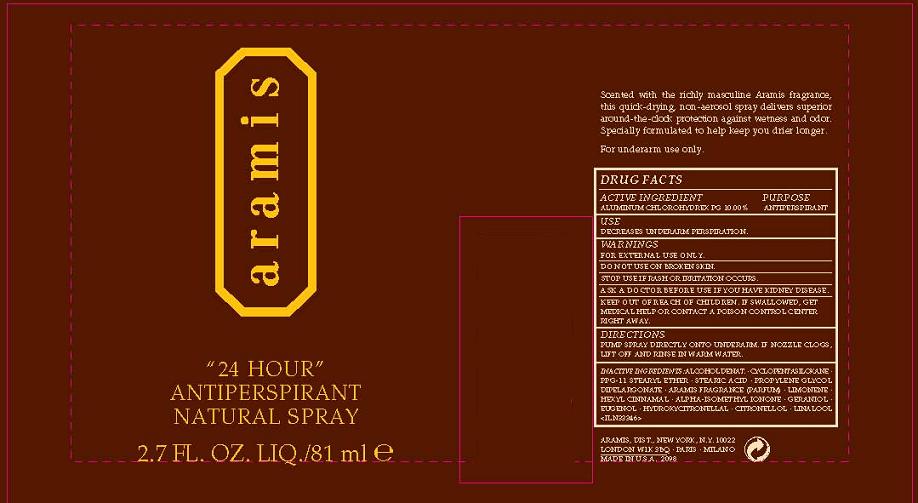
Label: 24 HOUR- aluminum zirconium tetrachlorohydrex gly spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 42472-001-01 - Packager: ARAMIS INC.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 5, 2011
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR/PHARMACIST
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
24 HOUR ANTIPERSPIRANT NATURAL
aluminum zirconium tetrachlorohydrex gly sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42472-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDREX PROPYLENE GLYCOL (UNII: PGG6VG42HP) (ALUMINUM CATION - UNII:3XHB1D032B) ALUMINUM CHLOROHYDREX PROPYLENE GLYCOL 10 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) STEARIC ACID (UNII: 4ELV7Z65AP) PROPYLENE GLYCOL DIETHYLHEXANOATE (UNII: 8D8I9Z0F1Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42472-001-01 81 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 10/01/1979 Labeler - ARAMIS INC. (042918826) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 205952385 manufacture Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 manufacture Establishment Name Address ID/FEI Business Operations Len-Ron Manufacturing Division of Aramis Inc. 809771152 manufacture Establishment Name Address ID/FEI Business Operations Aramis Inc. 042918826 manufacture Establishment Name Address ID/FEI Business Operations Northtec Bristol 949264774 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Northtec Keystone 618107429 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Pennsylvania Distribution Center 2 828534516 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd. 255175580 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd 253616536 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Distribution Center 208579636 repack, relabel Establishment Name Address ID/FEI Business Operations Estee Lauder Kabushiki Kaisha 712808195 relabel, repack Establishment Name Address ID/FEI Business Operations Whitman Laboratories Ltd. 216866277 manufacture Establishment Name Address ID/FEI Business Operations Aveda Corporation 071352058 manufacture Establishment Name Address ID/FEI Business Operations STANDARD SOAP 490709094 manufacture Establishment Name Address ID/FEI Business Operations SWALLOWFIELD PLC 296491541 manufacture