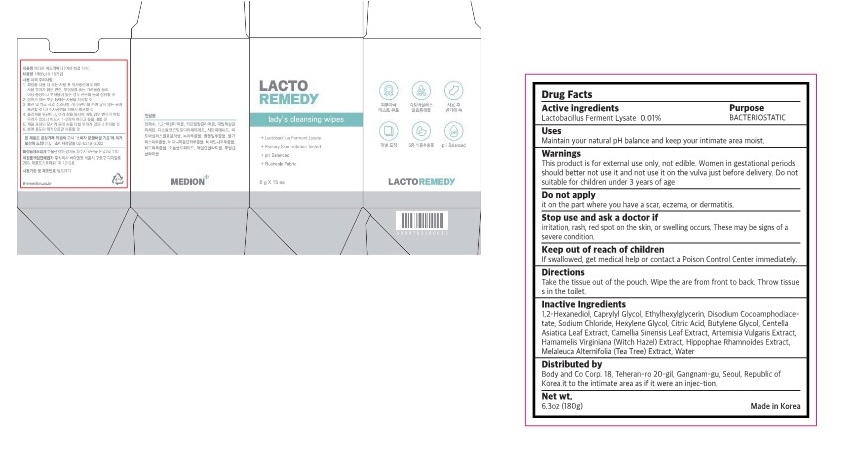
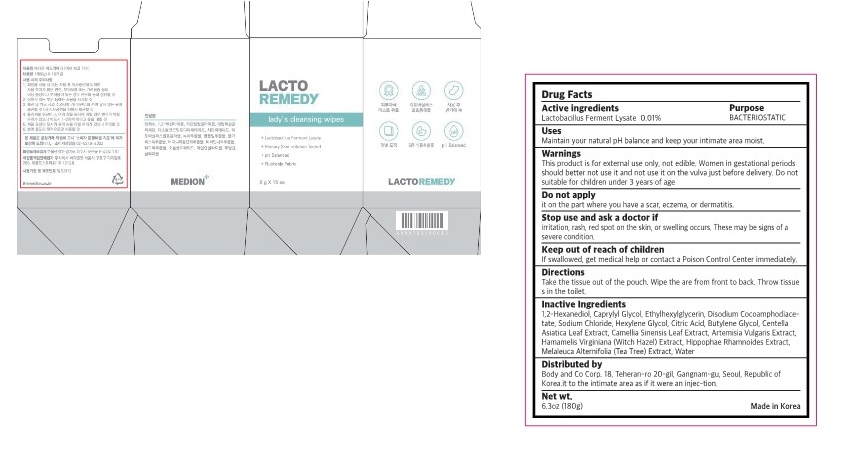
Label: LACTO REMEDY LADYS CLEANSING WIPES- lactobacillus ferment lysate swab
- NDC Code(s): 82892-302-01
- Packager: Body and Co Corp.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- purpose
- use
- warnings
- warnings
- warnings
- warnings
- Directions
-
Inactive ingredient
1,2-Hexanediol, Caprylyl Glycol, Ethylhexylglycerin, Disodium Cocoamphodiace- tate, Sodium Chloride, Hexylene Glycol, Citric Acid, Butylene Glycol, Centella Asiatica Leaf Extract, Camellia Sinensis Leaf Extract, Artemisia Vulgaris Extract, Hamamelis Virginiana (Witch Hazel) Extract, Hippophae Rhamnoides Extract, Melaleuca Alternifolia (Tea Tree) Extract, Water
- warnings
- label
-
INGREDIENTS AND APPEARANCE
LACTO REMEDY LADYS CLEANSING WIPES
lactobacillus ferment lysate swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82892-302 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIMOSILACTOBACILLUS FERMENTUM (UNII: BQX4W45LG8) (LIMOSILACTOBACILLUS FERMENTUM - UNII:BQX4W45LG8) LIMOSILACTOBACILLUS FERMENTUM 0.01 g in 100 g Inactive Ingredients Ingredient Name Strength HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) HIPPOPHAE RHAMNOIDES FRUIT (UNII: AVL0R9111T) HEXYLENE GLYCOL (UNII: KEH0A3F75J) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) SODIUM CHLORIDE (UNII: 451W47IQ8X) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CENTELLA ASIATICA LEAF (UNII: 6810070TYD) ARTEMISIA VULGARIS WHOLE (UNII: JDR81QW9ZQ) MELALEUCA ALTERNIFOLIA WHOLE (UNII: 976KV4FXYF) WATER (UNII: 059QF0KO0R) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82892-302-01 90 g in 1 BOX, UNIT-DOSE; Type 0: Not a Combination Product 07/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/26/2022 Labeler - Body and Co Corp. (695702369)