ADENOSCAN- adenosine solution
Astellas Pharma US, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADENOSCAN safely and effectively. See full prescribing information for ADENOSCAN.
ADENOSCAN® (adenosine) injection, for intravenous use Initial U.S. Approval: 1995 RECENT MAJOR CHANGESINDICATIONS AND USAGEAdenoscan, a pharmacologic stress agent, is indicated as an adjunct to thallium-201 myocardial perfusion scintigraphy in patients unable to exercise adequately (1) DOSAGE AND ADMINISTRATIONRecommended dose is 0.14 mg/kg/min infused over six minutes as a continuous peripheral intravenous infusion (total dose of 0.84 mg/kg) (2) DOSAGE FORMS AND STRENGTHSFor Injection: 3 mg/mL in single-dose vials (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence ≥ 10%) are: flushing; chest discomfort; shortness of breath; headache; throat, neck or jaw discomfort; gastrointestinal discomfort; and dizziness (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Astellas Pharma US, Inc. at 1-800-727-7003 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 4/2022 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Adenoscan (adenosine) is indicated as an adjunct to thallium-201 myocardial perfusion scintigraphy in patients unable to exercise adequately.
2 DOSAGE AND ADMINISTRATION
The recommended Adenoscan dose is 0.14 mg/kg/min infused over six minutes (total dose of 0.84 mg/kg) (Table 1).
- •
- Administer Adenoscan only as a continuous peripheral intravenous infusion
- •
- Inject Thallium-201 at the midpoint of the Adenoscan infusion (i.e., after the first three minutes of Adenoscan)
- •
- Thallium-201 is physically compatible with Adenoscan and may be injected directly into the Adenoscan infusion set
- •
- Inject Thallium-201 as close to the venous access as possible to prevent an inadvertent increase in the dose of Adenoscan (the contents of the intravenous tubing) being administered
Visually inspect Adenoscan for particulate matter and discoloration prior to administration. Do not administer Adenoscan if it contains particulate matter or is discolored.
There are no data on the safety or efficacy of alternative Adenoscan infusion protocols. The safety and efficacy of Adenoscan administered by the intracoronary route have not been established.
|
Patient Weight (kilograms) |
Infusion Rate
(mL per minute over 6
|
|
45 |
2.1 |
|
50 |
2.3 |
|
55 |
2.6 |
|
60 |
2.8 |
|
65 |
3 |
|
70 |
3.3 |
|
75 |
3.5 |
|
80 |
3.8 |
|
85 |
4 |
|
90 |
4.2 |
The nomogram displayed in Table 1 was derived from the following general formula:
- 0.14 (mg/kg/min) x
total body weight (kg) = Infusion rate
Adenoscan concentration (mL/min)
(3 mg/mL)
3 DOSAGE FORMS AND STRENGTHS
Adenoscan for injection is supplied as 20 mL and 30 mL single-dose vials containing a sterile, nonpyrogenic, clear solution of adenosine 3 mg/mL.
4 CONTRAINDICATIONS
Adenoscan is contraindicated in patients with:
- •
- Second- or third-degree AV block (except in patients with a functioning artificial pacemaker) [see Warnings and Precautions (5.2)]
- •
- Sinus node disease, such as sick sinus syndrome or symptomatic bradycardia (except in patients with a functioning artificial pacemaker) [see Warnings and Precautions (5.2)]
- •
- Known or suspected bronchoconstrictive or bronchospastic lung disease (e.g., asthma) [see Warnings and Precautions (5.3)]
- •
- Known hypersensitivity to Adenoscan [see Warnings and Precautions (5.7)]
5 WARNINGS AND PRECAUTIONS
5.1 Cardiac Arrest, Ventricular Arrhythmias, and Myocardial Infarction
Fatal and nonfatal cardiac arrest, sustained ventricular tachycardia (requiring resuscitation), and myocardial infarction have occurred following Adenoscan infusion. Avoid use in patients with symptoms or signs of acute myocardial ischemia, for example, unstable angina or cardiovascular instability; these patients may be at greater risk of serious cardiovascular reactions to Adenoscan. Appropriate resuscitative measures should be available [see Overdosage (10)].
5.2 Sinoatrial and Atrioventricular Nodal Block
Adenoscan exerts a direct depressant effect on the SA and AV nodes and may cause first-, second- or third-degree AV block, or sinus bradycardia. In clinical trials, approximately 6% of patients developed AV block following Adenoscan administration (first-degree heart block developed in 3%, second-degree in 3%, and third-degree in 0.8% of patients) [see Clinical Trials Experience (6.1)].
Use Adenoscan with caution in patients with pre-existing first-degree AV block or bundle branch block. Do not use in patients with high-grade AV block or sinus node dysfunction (except in patients with a functioning artificial pacemaker). Discontinue Adenoscan in any patient who develops persistent or symptomatic high-grade AV block.
5.3 Bronchoconstriction
Adenoscan administration can cause dyspnea, bronchoconstriction, and respiratory compromise. Adenoscan should be used with caution in patients with obstructive lung disease not associated with bronchoconstriction (e.g., emphysema, bronchitis). Do not use in patients with bronchoconstriction or bronchospasm (e.g., asthma). Discontinue Adenoscan in any patient who develops severe respiratory difficulties. Resuscitative measures should be available prior to Adenoscan administration [see Clinical Trials Experience (6.1), Overdosage (10), and Clinical Pharmacology (12.2)].
5.4 Hypotension
Adenoscan is a potent peripheral vasodilator and can induce significant hypotension. The risk of serious hypotension may be higher in patients with autonomic dysfunction, hypovolemia, stenotic valvular heart disease, pericarditis or pericardial effusions, or stenotic carotid artery disease with cerebrovascular insufficiency. Discontinue Adenoscan in any patient who develops persistent or symptomatic hypotension.
5.5 Cerebrovascular Accident
Hemorrhagic and ischemic cerebrovascular accidents have occurred. Hemodynamic effects of Adenoscan including hypotension or hypertension can be associated with these adverse reactions [see Warnings and Precautions (5.4) and (5.9)].
5.6 Seizures
New-onset or recurrence of convulsive seizures has occurred following Adenoscan. Some seizures are prolonged and require emergent anticonvulsive management. Aminophylline may increase the risk of seizures associated with Adenoscan. Methylxanthine use is not recommended in patients who experience seizures in association with Adenoscan administration [see Overdosage (10)].
5.7 Hypersensitivity
Dyspnea, throat tightness, flushing, erythema, rash, and chest discomfort have occurred. Symptomatic treatment may be required. Have personnel and appropriate treatment available. Resuscitative measures may be necessary if symptoms progress [see Post-Marketing Experience (6.2)].
5.8 Atrial Fibrillation
Adenoscan can cause atrial fibrillation in patients with or without a history of atrial fibrillation. Atrial fibrillation typically began 1.5 to 3 minutes after initiation of Adenoscan, lasted for 15 seconds to 6 hours, and spontaneously converted to normal sinus rhythm [see Post-Marketing Experience (6.2)].
5.9 Hypertension
Adenoscan can induce clinically significant increases in systolic and diastolic blood pressure. Most increases resolved spontaneously within several minutes, but in some cases, hypertension lasted for several hours [see Clinical Trials Experience (6.1)].
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the prescribing information:
- •
- Fatal Cardiac Arrest, Ventricular Arrhythmias, and Myocardial Infarction [see Warnings and Precautions (5.1)]
- •
- Sinoatrial and Atrioventricular Nodal Block [see Warnings and Precautions (5.2)]
- •
- Bronchoconstriction [see Warnings and Precautions (5.3)]
- •
- Hypotension [see Warnings and Precautions (5.4)]
- •
- Cerebrovascular Accident [see Warnings and Precautions (5.5)]
- •
- Seizures [see Warnings and Precautions (5.6)]
- •
- Hypersensitivity [see Warnings and Precautions (5.7)]
- •
- Atrial Fibrillation [see Warnings and Precautions (5.8)]
- •
- Hypertension [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions, with an incidence of at least 1%, were reported with Adenoscan among 1,421 patients in clinical trials. Eleven percent (11%) of the adverse reactions occurred several hours after Adenoscan administration. Eight percent (8%) of the adverse reactions began with Adenoscan infusion and persisted for up to 24 hours.
The most common (incidence ≥ 10%) adverse reactions to Adenoscan are flushing, chest discomfort, shortness of breath, headache, throat, neck or jaw discomfort, gastrointestinal discomfort, and dizziness (Table 2).
| Adverse Reactions | Adenoscan
N=1,421 |
|---|---|
|
Flushing |
44% |
|
Chest discomfort |
40% |
|
Dyspnea |
28% |
|
Headache |
18% |
|
Throat, neck or jaw discomfort |
15% |
|
Gastrointestinal discomfort |
13% |
|
Lightheadedness/dizziness |
12% |
|
Upper extremity discomfort |
4% |
|
ST segment depression |
3% |
|
First-degree AV block |
3% |
|
Second-degree AV block |
3% |
|
Paresthesia |
2% |
|
Hypotension |
2% |
|
Nervousness |
2% |
|
Arrhythmias |
1% |
Adverse reactions to Adenoscan of any severity reported in less than 1% of patients include:
- Body as a Whole: back discomfort, lower extremity discomfort, weakness
Cardiovascular System: myocardial infarction, ventricular arrhythmia, third-degree AV block,
bradycardia, palpitation, sinus exit block, sinus pause, T-wave changes,
hypertension (systolic blood pressure > 200 mm Hg)
Respiratory System: cough
Central Nervous System: drowsiness, emotional instability, tremors
Genital/Urinary System: vaginal pressure, urgency
Special Senses: blurred vision, dry mouth, ear discomfort, metallic taste, nasal
congestion, scotomas, tongue discomfort
6.2 Post-Marketing Experience
The following adverse reactions have been reported from marketing experience with Adenoscan. Because these reactions are reported voluntarily from a population of uncertain size, are associated with concomitant diseases and multiple drug therapies and surgical procedures, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders: cardiac arrest, atrial fibrillation, cardiac failure,
myocardial infarction, tachycardia, ventricular arrhythmia
Gastrointestinal Disorders: nausea and vomiting
General Disorders and Administration Site Conditions: chest pain, injection site reaction, infusion site pain
Immune System Disorders: hypersensitivity
Nervous System Disorders: cerebrovascular accident including intracranial hemorrhage,
seizure activity including tonic-clonic (grand mal) seizures, loss
of consciousness
Respiratory, Thoracic and Mediastinal Disorders: bronchospasm, respiratory arrest, throat tightness
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Adenoscan
- •
- The vasoactive effects of adenosine are inhibited by adenosine receptor antagonists, such as methylxanthines (e.g., caffeine, aminophylline, and theophylline). The safety and efficacy of Adenoscan in the presence of these agents has not been systematically evaluated [see Overdosage (10)].
- •
- The vasoactive effects of Adenoscan are potentiated by nucleoside transport inhibitors such as dipyridamole. The safety and efficacy of adenosine in the presence of dipyridamole has not been systematically evaluated.
- •
- Whenever possible, drugs that might inhibit or augment the effects of adenosine should be withheld for at least five half-lives prior to the use of Adenoscan.
7.2 Effects of Adenoscan on Other Drugs
Adenoscan adenosine injection has been given with other cardioactive drugs (such as beta adrenergic blocking agents, cardiac glycosides, and calcium channel blockers) without apparent adverse interactions, but its effectiveness with these agents has not been systematically evaluated. Because of the potential for additive or synergistic depressant effects on the SA and AV nodes, however, Adenoscan should be used with caution in the presence of these agents [see Warnings and Precautions (5.2)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with adenosine; nor have studies been performed in pregnant women. Because it is not known whether Adenoscan can cause fetal harm when administered to pregnant women, Adenoscan should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
It is not known whether Adenoscan is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from Adenoscan in nursing infants, the decision to interrupt nursing after administration of Adenoscan or not to administer Adenoscan, should take into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of Adenoscan in patients less than 18 years of age have not been established.
8.5 Geriatric Use
Clinical studies with Adenoscan did not include sufficient numbers of subjects aged younger than 65 years to determine whether they respond differently. Other reported experience has not revealed clinically relevant differences of the response of elderly in comparison to younger patients.
10 OVERDOSAGE
The half-life of adenosine is less than 10 seconds and adverse reactions of Adenoscan usually resolve quickly when the infusion is discontinued, although delayed or persistent reactions have been observed. Methylxanthines, such as caffeine, aminophylline, and theophylline, are competitive adenosine receptor antagonists and theophylline has been used to terminate persistent adverse reactions. In clinical trials, theophylline (50-125 mg slow intravenous injection) was used to attenuate Adenoscan adverse reactions in approximately 2% of patients. Methylxanthine use is not recommended in patients who experience seizures in association with Adenoscan [see Drug Interactions (7.1)].
11 DESCRIPTION
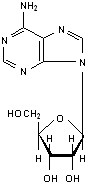
Adenosine is an endogenous nucleoside and is chemically described as 6-amino-9-beta-D-ribofuranosyl-9-H-purine. Adenosine has the following structural formula:
The molecular formula for adenosine is C10H13N5O4 and its molecular weight is 267.24.
Adenosine is a white crystalline powder. It is soluble in water and practically insoluble in alcohol. Solubility increases by warming and lowering the pH of the solution.
Each Adenoscan vial contains a sterile, non-pyrogenic solution of adenosine 3 mg/mL and sodium chloride 9 mg/mL in water for injection, with pH between 4.5 and 7.5.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Adenosine causes cardiac vasodilation which increases cardiac blood flow. Adenosine is thought to exert its pharmacological effects through activation of purine receptors (cell-surface A1 and A2 adenosine receptors). Although the exact mechanism by which adenosine receptor activation relaxes vascular smooth muscle is not known, there is evidence to support both inhibition of the slow inward calcium current reducing calcium uptake, and activation of adenylate cyclase through A2 receptors in smooth muscle cells. Adenosine may also lessen vascular tone by modulating sympathetic neurotransmission. The intracellular uptake of adenosine is mediated by a specific transmembrane nucleoside transport system. Once inside the cell, adenosine is rapidly phosphorylated by adenosine kinase to adenosine monophosphate, or deaminated by adenosine deaminase to inosine. These intracellular metabolites of adenosine are not vasoactive.
Myocardial uptake of thallium-201 is directly proportional to coronary blood flow. Since Adenoscan significantly increases blood flow in normal coronary arteries with little or no increase in stenotic arteries, Adenoscan causes relatively less thallium-201 uptake in vascular territories supplied by stenotic coronary arteries (i.e., a greater difference is seen after Adenoscan between areas served by normal and areas served by stenotic vessels than is seen prior to Adenoscan).
12.2 Pharmacodynamics
Hemodynamic Effects
Adenosine produces a direct negative chronotropic, dromotropic and inotropic effect on the heart, presumably due to A1-receptor agonism, and produces peripheral vasodilation, presumably due to A2-receptor agonism. The net effect of Adenoscan in humans is typically a mild to moderate reduction in systolic, diastolic and mean arterial blood pressure associated with a reflex increase in heart rate. Rarely, significant hypotension and tachycardia have been observed [see Warnings and Precautions (5.4)].
12.3 Pharmacokinetics
Distribution
Intravenously administered adenosine distributes from the circulation via cellular uptake, primarily by erythrocytes and vascular endothelial cells. This process involves a specific transmembrane nucleoside carrier system that is reversible, nonconcentrative, and bidirectionally symmetrical.
Metabolism
Intracellular adenosine is metabolized either via phosphorylation to adenosine monophosphate by adenosine kinase, or via deamination to inosine by adenosine deaminase in the cytosol. Since adenosine kinase has a lower Km and Vmax than adenosine deaminase, deamination plays a significant role only when cytosolic adenosine saturates the phosphorylation pathway. Inosine formed by deamination of adenosine can leave the cell intact or can be degraded to hypoxanthine, xanthine, and ultimately uric acid. Adenosine monophosphate formed by phosphorylation of adenosine is incorporated into the high-energy phosphate pool.
Elimination
While extracellular adenosine is primarily cleared from plasma by cellular uptake with a half-life of less than 10 seconds in whole blood, excessive amounts may be deaminated by an ecto-form of adenosine deaminase.
Specific Populations
Renal Impairment
As adenosine does not require renal function for its activation or inactivation, renal impairment would not be expected to alter its effectiveness or tolerability.
Hepatic Impairment
As adenosine does not require hepatic function for its activation or inactivation, hepatic impairment would not be expected to alter its effectiveness or tolerability.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals have not been performed to evaluate adenosine’s carcinogenic potential or potential effects on fertility. Adenosine was negative for genotoxic potential in the Salmonella (Ames Test) and Mammalian Microsome Assay.
Adenosine, however, like other nucleosides at millimolar concentrations present for several doubling times of cells in culture, is known to produce a variety of chromosomal alterations.
14 CLINICAL STUDIES
In two crossover comparative studies involving 319 subjects who could exercise (including 106 healthy volunteers and 213 patients with known or suspected coronary disease), Adenoscan and exercise thallium images were compared by blinded observers. The images were concordant for the presence of perfusion defects in 85.5% of cases by global analysis (patient by patient) and up to 93% of cases based on vascular territories.
In the two studies, 193 patients also had recent coronary arteriography for comparison (healthy volunteers were not catheterized). The sensitivity for detecting angiographically significant disease (≥ 50% reduction in the luminal diameter of at least one major vessel) was 64% for Adenoscan and 64% for exercise testing. The specificity was 54% for Adenoscan and 65% for exercise testing. The 95% confidence limits for Adenoscan sensitivity were 56% to 78% and for specificity were 37% to 71%.
Intracoronary Doppler flow catheter studies have demonstrated that a dose of intravenous Adenoscan of 0.14 mg/kg/min produces maximum coronary hyperemia (relative to intracoronary papaverine) in approximately 95% of cases within two to three minutes of the onset of the infusion. Coronary blood flow velocity returns to basal levels within one to two minutes of discontinuing the Adenoscan infusion.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Adenoscan is supplied as 20 mL and 30 mL vials of sterile, nonpyrogenic, preservative-free, solution in normal saline:
- •
-
20 mL single-dose vial: NDC 0469-0871-20
60 mg/20 mL (3 mg/mL) in a 20 mL single-dose, flip-top glass vial, packaged individually and in packages of ten - •
-
30 mL single-dose vial: NDC 0469-0871-30
90 mg/30 mL (3 mg/mL) in a 30 mL single-dose, flip-top glass vial, packaged individually and in packages of ten
17 PATIENT COUNSELING INFORMATION
- •
- Advise patients that they may be at increased risk of fatal and nonfatal heart attacks, abnormal heart rhythms, cardiac arrest, heart block, significant increase or decrease in blood pressure, bronchoconstriction, hypersensitivity reactions, seizures, or cerebrovascular accidents with the use of Adenoscan [see Warnings and Precautions (5.1-5.9)].
- •
- Advise patients with COPD or asthma to discuss their respiratory history with their clinician before scheduling a myocardial perfusion imaging study with Adenoscan [see Warnings and Precautions (5.3)].
- •
- Methylxanthines have the potential to impact the effects of Adenoscan. Instruct patients to avoid consumption of any products containing methylxanthines, including caffeinated coffee, tea or other caffeinated beverages, caffeine-containing drug products, aminophylline, and theophylline prior to the myocardial perfusion imaging study. Question patients about a history of seizures [see Warnings and Precautions (5.6), Drug Interactions (7.1), and Overdosage (10)].
Rx only
Product of Germany
Marketed by:
Astellas Pharma US, Inc.
Northbrook, IL 60062 USA
Manufactured by:
Hospira, Inc.
Lake Forest, IL 60045 USA
14H059-ADS
© 2014 Astellas Pharma US, Inc.
ADENOSCAN® is a registered trademark of Astellas US LLC.
| ADENOSCAN
adenosine solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Astellas Pharma US, Inc. (605764828) |