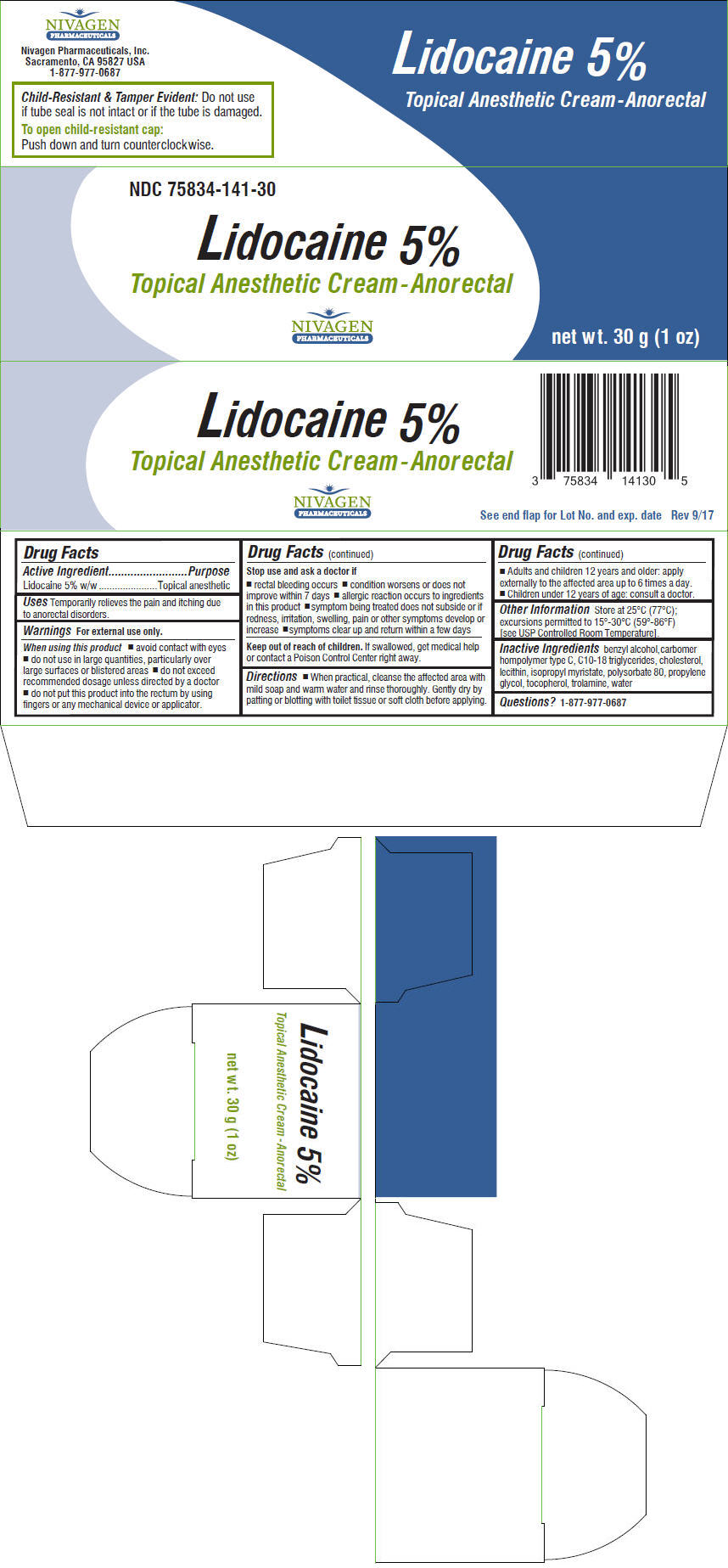
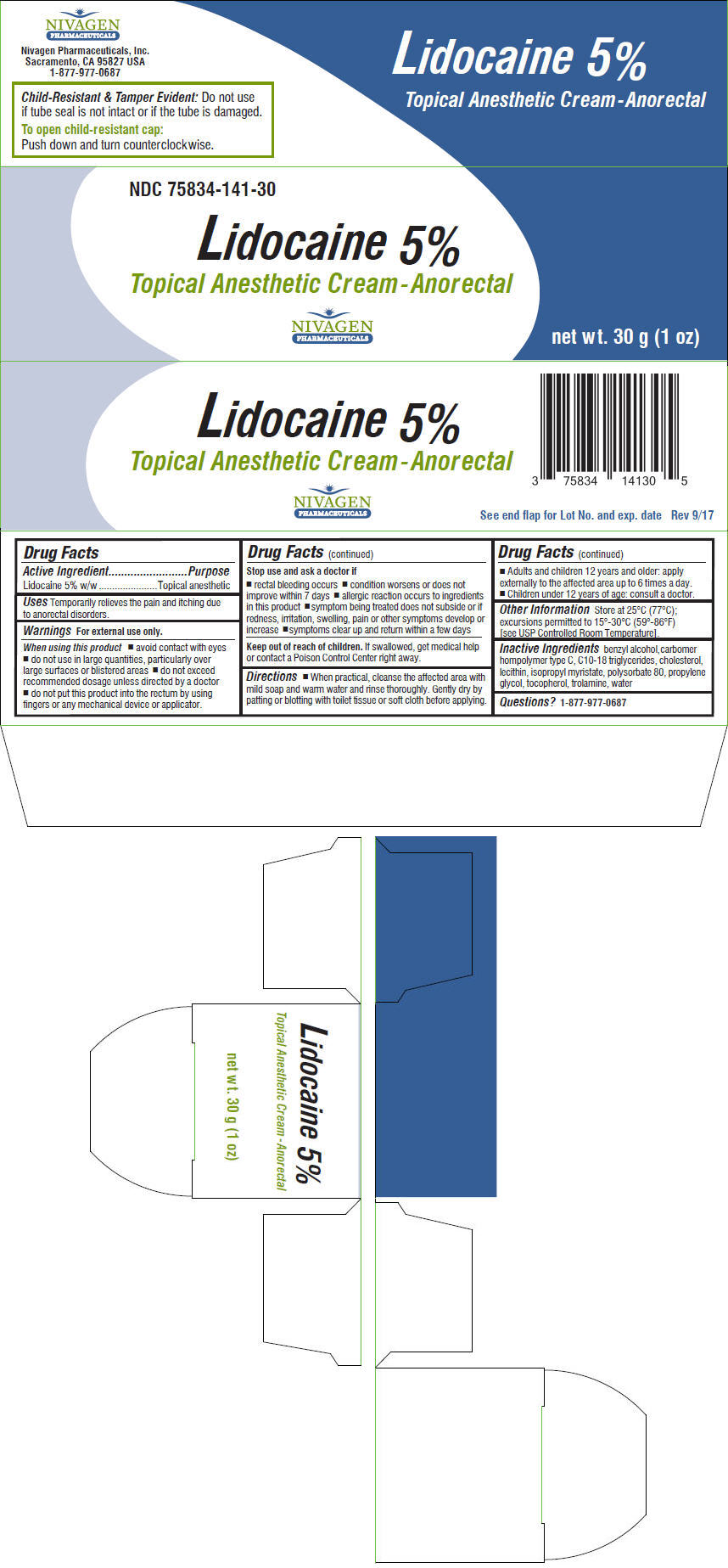
Label: LIDOCAINE cream
- NDC Code(s): 75834-141-30
- Packager: Nivagen Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only.
When using this product
- avoid contact with eyes
- do not use in large quantities, particularly over large surfaces or blistered areas
- do not exceed recommended dosage unless directed by a doctor
- do not put this product into the rectum by using fingers or any mechanical device or applicator.
Stop use and ask a doctor if
- rectal bleeding occurs
- condition worsens or does not improve within 7 days
- allergic reaction occurs to ingredients in this product
- symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase
- symptoms clear up and return within a few days
-
Directions
- When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or soft cloth before applying.
- Adults and children 12 years and older: apply externally to the affected area up to 6 times a day.
- Children under 12 years of age: consult a doctor.
- Other Information
- Inactive Ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 30 g Tube Carton
-
INGREDIENTS AND APPEARANCE
LIDOCAINE
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75834-141 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CHOLESTEROL (UNII: 97C5T2UQ7J) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TOCOPHEROL (UNII: R0ZB2556P8) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-141-30 30 g in 1 TUBE; Type 0: Not a Combination Product 12/15/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/15/2016 Labeler - Nivagen Pharmaceuticals, Inc. (052032418) Registrant - Westwood Laboratories, LLC (832280635)