Label: ERYTHROMYCIN ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 55045-1350-9 - Packager: Dispensing Solutions, Inc.
- This is a repackaged label.
- Source NDC Code(s): 17478-070
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 22, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
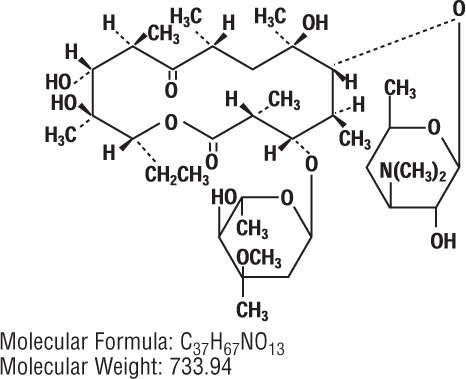
Erythromycin Ophthalmic Ointment belongs to the macrolide group of antibiotics. It is basic and readily forms a salt when combined with an acid. The base, as crystals or powder, is slightly soluble in water, moderately soluble in ether, and readily soluble in alcohol or chloroform. Erythromycin ((3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)-oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethyl-amino)-β-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione)) is an antibiotic produced from a strain of Streptomyces erythraeus.
It has the following structural formula:
Each gram contains Erythromycin USP 5 mg in a sterile ophthalmic base of mineral oil and white petrolatum.
-
CLINICAL PHARMACOLOGY:
Microbiology: Erythromycin inhibits protein synthesis without affecting nucleic acid synthesis. Erythromycin is usually active against the following organisms in vitro and in clinical infections:
Streptococcus pyogenes (group A β-hemolytic)
Alpha-hemolytic streptococci (viridans group)
Staphylococcus aureus, including penicillinase-producing strains (methicillin-resistant staphylococci are uniformly resistant to erythromycin)
Streptococcus pneumoniae
Mycoplasma pneumoniae (Eaton Agent, PPLO)
Haemophilus influenzae (not all strains of this organism are susceptible at the erythromycin concentrations ordinarily achieved)
Treponema pallidum
Corynebacterium diphtheriae
Neisseria gonorrhoeae
Chlamydia trachomatis
-
INDICATIONS AND USAGE:
For the treatment of superficial ocular infections involving the conjunctiva and/or cornea caused by organisms susceptible to erythromycin.
For prophylaxis of ophthalmia neonatorum due to N. gonorrhoeae or C. trachomatis.
The effectiveness of erythromycin in the prevention of ophthalmia caused by penicillinase-producing N.gonorrhoeae is not established.
For infants born to mothers with clinically apparent gonorrhea, intravenous or intramuscular injections of aqueous crystalline penicillin G should be given; a single dose of 50,000 units for term infants or 20,000 units for infants of low birth weight. Topical prophylaxis alone is inadequate for these infants.
- CONTRAINDICATIONS:
-
PRECAUTIONS:
General: The use of antimicrobial agents may be associated with the overgrowth of nonsusceptible organisms including fungi; in such a case, antibiotic administration should be stopped and appropriate measures taken.
Information for Patients: Avoid contaminating the applicator tip with material from the eye, fingers, or other source.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Two year oral studies conducted in rats with erythromycin did not provide evidence of tumorigenicity. Mutagenicity studies have not been conducted. No evidence of impaired fertility that appeared related to erythromycin was reported in animal studies.
Pregnancy: Teratogenic Effects: Pregnancy Category B. Reproduction studies have been performed in rats, mice, and rabbits using erythromycin and its various salts and esters, at doses that were several multiples of the usual human dose. No evidence of harm to the fetus that appeared related to erythromycin was reported in these studies. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, the erythromycins should be used during pregnancy only if clearly needed.
- ADVERSE REACTIONS:
-
DOSAGE AND ADMINISTRATION:
In the treatment of superficial ocular infections, a ribbon approximately 1 cm in length of Erythromycin Opthalmic Ointment should be applied directly to the infected structure up to 6 times daily, depending on the severity of the infection.
For prophylaxis of neonatal gonococcal or chlamydial conjunctivitis, a ribbon of ointment approximately 1 cm in length should be instilled into each lower conjunctival sac. The ointment should not be flushed from the eye following instillation. A new tube should be used for each infant.
- HOW SUPPLIED:
- Principal Display Panel Text for Container Label
-
INGREDIENTS AND APPEARANCE
ERYTHROMYCIN
erythromycin ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55045-1350(NDC:17478-070) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Erythromycin (UNII: 63937KV33D) (Erythromycin - UNII:63937KV33D) Erythromycin 5 mg in 1 g Inactive Ingredients Ingredient Name Strength mineral oil (UNII: T5L8T28FGP) petrolatum (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55045-1350-9 1 in 1 CARTON 1 3.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA064030 07/18/1996 Labeler - Dispensing Solutions, Inc. (066070785) Establishment Name Address ID/FEI Business Operations Dispensing Solutions, Inc. 066070785 relabel, repack